Institutional Annual/Final Research Report
advertisement

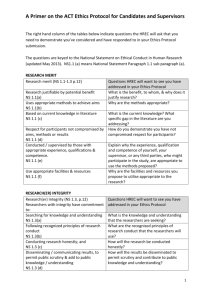
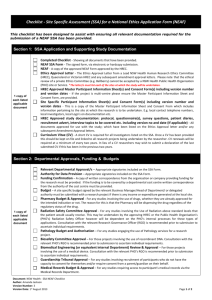
Northern Sydney Local Health District (NSLHD) Annual/Final Research Report to Institution 1. Submission of Annual Reports is a condition of ongoing ethics and/or governance approval. Reports are due every year on the 30 August until the study is completed and a summary of results of available. 2. For multi-centre studies with lead ethics approval by NSLHD HREC, one report (incorporating information relating to any sub-studies conducted under the same file number) should be submitted by the Coordinating Investigator (a separate report to the NSLHD Research Governance Officer is not required) 3. For studies with lead ethics approval from an HREC other than NSLHD HREC (ie. governance only), this report must be completed in addition to any reports which must be submitted to the lead HREC. 4. This form should be filled in electronically and emailed to the Research Office. One hard copy must also be submitted. Electronic signatures are acceptable, however, if a study coordinator is submitting on behalf of the Principal Investigator (PI), the PI must be cc’d on any correspondence. 5. YOU MAY NOT NEED TO COMPLETE ALL SECTIONS OF THIS REPORT. DELETE SECTIONS THAT DO NOT APPLY TO YOUR STUDY A. Administrative details: complete for all reports B. PROGRESS SUMMARY B: Complete for studies which involve participant recruitment PROGRESS SUMMARY B.1: Complete for studies which involve tissue banking C. PROGRESS SUMMARY C: Complete for studies which do not involve direct participant recruitment (use of banked tissue and/or data banking, anonymous surveys, medical record review etc) D. PROGRESS SUMMARY D: Complete for all clinical trials, or other studies sponsored by a commercial entity or collaborative research group E. FINAL REPORT: complete for all completed studies, in addition to other relevant sections This report, with Coordinating/Principal Investigator signature (electronic or scanned) must be emailed to NSLHD-Research@health.nsw.gov.au. A 1 Please also submit one hard copy: Post: Research Office Level 13, Kolling Building Royal North Shore Hospital St Leonards NSW 2065 In person Kolling Building level 4 (ground floor lobby) ADMINISTRATIVE DETAILS: complete for all projects What type of report is this? Annual ☐ Section E cannot be left blank if this Final ☐ is a final report 2 3 4 5 6 7 8 B 1 2 NSLHD File Number eg. RESP/xx/xx or xxxx-xxxM HREC Reference number eg. HREC/year/HAWKE/xxx Project title HREC approval expiry date Coordinating/Principal Investigator Current status at all sites approved by NSLHD HREC or RGO Investigator name at site Site name Current status (active, not yet active, complete, terminated, abandoned) If a site has not yet commenced, or has been terminated/abandoned, please provide the reason. Please confirm that any personnel changes in the last 12 months have been submitted for HREC and/or RGO review ☐Yes – all notifications submitted previously ☐Yes – submitted with this report (completed per instructions on Change in Personnel Form) ☐No – no changes in personnel in last 12 months PROGRESS SUMMARY B: Complete for studies which involve participant recruitment (please list information for all NSLHD sites, AND, if applicable, sites approved by NSLHD HREC) Date of first patient recruited at NSLHD Is recruitment on target per the anticipated number in the Site Specific Assessment and/or feasibility assessment? NSLHD Institutional Research Report – July 2015 v1 DELAYED ☐- provide reason for delay Page 1 of 4 ON TARGET COMPLETE 3 4 5 ☐ ☐ Total number of participants enrolled (by site, if applicable) Number of participants withdrawn from study (provide reason for withdrawal) Number of participants screened but not enrolled Does the study involve tissue banking? 6 ☐No – delete section B1 ☐Yes – storage and use of tissue is dealt with in study contracts (eg. formal commercial or collaborative group CTRA is in place) DELETE section B1 ☐Yes – study without formal contracts which cover tissue use/banking COMPLETE section B1 B1 1 2 TISSUE BANKING Number of specimens collected to-date Location at which specimens are stored Have the specimens been used for other research projects? 3 C 1 2 3 4 5 ☐No ☐Yes – please provide the following: HREC approval number for use of tissue (NSLHD reference #, or if not approved by NSLHD HREC, attach relevant HREC approval letter) Copy of Material Transfer Agreement (if specimens transferred to another institution) Number of specimens used to-date PROGRESS SUMMARY C - Complete for studies which do not involve direct participant recruitment (use of banked tissue and/or databases, medical record review etc) Please list information for all NSLHD sites, AND, if applicable, sites approved by NSLHD HREC Date study commenced at NSLHD Total number of records accessed in database OR Total number of specimens accessed Number of records screened prior to use in dataset or specimen use Source of data/tissue (eg. medical records, or approved research database/tissue bank – specify which database/tissue bank) Specific location at which data is stored/held (eg. Departmental computers on NSLHD servers, specific locked filing cupboard, University servers etc) 1 PROGRESS SUMMARY D - Complete for all clinical trials, or other studies sponsored by a commercial entity or collaborative research group Insurance certificate number and expiry date 2 Clinical trial registration number 3 Please confirm that all DSMB/safety monitoring reports from the last 12 months have been submitted ☐Yes – all reports submitted ☐No – submitted with this report ☐No – no safety/DSMB reports in last 12 months 4 If the study has been audited, either by the sponsor or by an external regulatory body, at any NSLHD sites (or sites under the jurisdiction of NSLHD HREC), please confirm that the audit report has been submitted ☐Not audited ☐Audited and report submitted previously ☐Audited, report submitted with this progress report 5 Please confirm that any interim analyses from the last 12 months have been submitted ☐Yes – all reports submitted ☐No – submitted with this report ☐No – no analyses conducted in last 12 months D NSLHD Institutional Research Report – July 2015 v1 Page 2 of 4 If N/A, delete this section - The final report should not be submitted if data E FINAL REPORT 1 Project completion date 2 Lay summary of findings analysis is ongoing. If a global study sponsor requires ongoing submission of regulatory documents for review by the HREC, even if a study has closed in Australia, do not submit a final report. Provide details of publications, presentations given or reports (including funding reports) accepted or in press 3 Archiving Period (per NSW State Records Act GDA17) Please note that these periods apply from the date of final publication or termination of the study 4 ☐Indefinite ☐15 years ☐5 years ☐3 years Interventional TGA/CTN Scheme; Gene Therapy (after 2000 – per Code for Responsible Conduct of Research) Interventional/Clinical research (GDA17 8.1.1) Non Interventional/Low Risk projects (GDA17 8.1.2) Other (eg. Project never commenced, projects were not approved, no participants enrolled) (GDA17 8.1.5) Select one option only F SIGN-OFF BY COORDINATING/PRINCIPAL INVESTIGATOR By signing the declaration below, you are confirming the following: 1. This project is being/has been conducted as originally approved by the relevant ethics committee (and subject to any changes subsequently approved as amendments) 2. This project continues to be conducted in compliance with the NHMRC National Statement on Ethical Conduct in Human Research (NHMRC, 2007) and relevant institutional requirements of NSLHD 3. All amendments have been submitted for HREC and/or RGO review prior to implementation 4. All relevant safety reports have been submitted for HREC and/or RGO review 5. All protocol deviations/violations have been reported (per NSW GL2010_014 section 11) 6. The HREC and/or RGO have been notified of any changed in personnel in the last 12 months 7. This report accurately reflects the progress of the project Name of Coordinating Investigator Signature of Coordinating Investigator Date Contact details for enquiries relating to this report: Name / phone / email NSLHD Research Office use only – HREC Date Reviewed by HREC Executive: NSLHD Institutional Research Report – July 2015 v1 Page 3 of 4 Action: Name: Signature: Research Ethics Manager | HREC Chair | Ethics Officer | RCGM NSLHD Research Office use only – RGO Date Reviewed by Research Governance Officer: Action: Name: Signature: Research Governance Officer | RCGM NSLHD Institutional Research Report – July 2015 v1 Page 4 of 4