File
advertisement

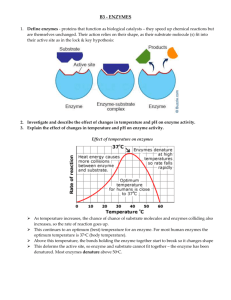
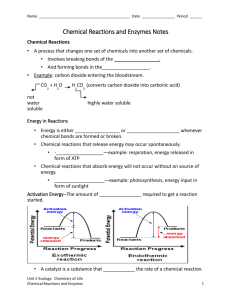
Enzyme stabilization by selection and genetic engineering Enzyme stabilization by selection Biocatalysts are inherently labile; therefore their operational stability is very importance for any bioprocess. An industrial disadvantage of the most commercially used biocatalysts- enzymes and enzyme complexes - is their relatively low stability. Selection of industrial enzymes from any sources is depends on the following factors: a. specificity b. pH c. thermostability d. activation or inhibition e. availability and cost Therefore, microorganisms which require extreme physicochemical conditions for their growth and proliferation - and enzymes derived from them - bear a potential for overcoming this situation. The particular, enzymes derived from thermophilic microorganisms show an increased thermal stability compared to those of mesophilic microorganisms. They are called thermostable if they have a maximum reaction temperature above that of the optimum growth temperature for the microorganism. This definition is invalid in view of the fact that enzymes from microorganisms growing at temperatures above 90 OC are "thermostable" regardless of whether their optimum activity temperature is below or above the optimum growth temperature. It becomes obvious that thermostability of an enzyme is a relative term. Stability of the enzyme is a function of the stabilizing forces which include hydrogen bonding, hydrophobic bonding, ionic interactions, metal binding, and/or disulfide linkages . Such stabilizing effects contribute to the long-term stability of an enzyme. Thermostability is also connected with a higher resistance to most chemical denaturants. A suitable method for characterizing the enzyme stability is the scanning calorimetry. Thermophilic microorganisms are a source of more stable enzymes. Mixed populations of bacteria which seemed to occur in the ocean near the Galapagos Island in a depth of 2500 metres were generally an optimum growth temperature is 250OC at a pressure of 250 atm . There is reliable verification of the existence of microorganisms at temperatures approaching the boiling point of water given in the (Table 1 ) Table 1. Optimum growth temperature ( Topt ) of thermophilic bacteria Species Topt ( OC) Bacillus acidocaldarius Bacillus stearothermophilus Caldarobacterium hydrogenophilum 60 - 65 55 - 70 74 - 76 Clostridium thermohydrosulfuricum Methanobacterium thermolithotrophicum Pyrococcus furiosus Pyrodictium occultum Sulfolobus acidocaldarius Thermoproteus tenax Thermotoga maritime Thermus aquaticus 67 - 70 65 - 70 100 105 70 - 75 88 80 70 These bacteria occur in natural and/or artificial habitats. As an example the original isolations of Thermus aquaticus were from hot spring algal mats. Incubation in aerobic liquid medium at 70 up to 75OC led to the formation of visible turbidity, often with clumps. Brock and Freeze isolated one strain of Thermus aquaticus from a hot water tap in Indiana. Brock and Yoder found another strain of Thermus aquaticus in a creek receiving thermal pollution. Ramely and Hixson as well as Brock and Boylen and Heinritz et al. obtained strains of Thermus aquaticus in a creek receiving thermal pollution and domestic hot water reservoirs, respectively. It thus seems likely that Thermus is capable of growing in manmade habitats of high temperature. Table 2 shows the specific growth rate and the specific yield coefficient of selected thermophilic bacteria utilizing glucose as the carbon and energy sources at temperatures up to 80OC. Table 2. Specific growth rate (μ) specific yield coefficient ( Y x/ s), and optimum growth temperature (& topt ) of selected thermophilic microorganisms utilizing glucose. From the table 2 we find that the specific growth rate is in the range of the values of mesophilic bacteria. On the other hand, the specific yield coefficient is obviously lower compared to mesophiles. The specific growth rate and/or the specific yield coefficient of thermophilic bacteria could be improved by - continuous instead of batch cultivation preventing inhibition of bacterial growth and proliferation due to carbon substrate and/or metabolites - increasing system pressure in fermenters. Advantage of More stable enzymes from thermophilic microorganisms Thermostable enzymes have several advantages over their counterparts from mesophilic microorganisms - higher thermal stability and resistance to most of the chemical denatu- higher storage stability, - increased reaction rate and comparable catalytic activity, - lower viscosity of reaction mixture and improved mass-transfer, - lower danger of contamination in microbial enzyme production as well as Fig 1: Thermal stability of a thermostable β-galactosidase of Bacillus stearothermophilus TP32 compared to the mesophilic β-galactosidase of Escherichia coli Fig 2: Resistance of the thermostable (β-galactosidase of Bacillus stearothermophilus TP 32 and the mesophilic β–galactosidase of Escherichia coli to ethanol and propan-2-ol under incubation of the enzyme for 60 min at 50OC Application of thermostable enzymes Because of these facts thermostable carbohydratases, proteases, and oxidoreductases were introduced recently into starch processing, hydrolysis of cellulose and lactose, brewing, baking, food processing, waste water treatment, biosensors and/or other applications. Thermostable and highly specific enzymes, e.g. DNA polymerases and RNA polymerases open up new dimensions in molecular biology and genetic engineering. As an example the DNA polymerase of Thermus aquaticus made possible the polymerase chain reaction ( PCR ) technology. Enzyme stabilization by genetic engineering Genetic engineering and protein engineering are modern techniques already in use for the commercial production of biocatalysts of improved stability, not only to high temperatures, but also to extremes of pH, oxidizing agents and organic solvents. Cloning and expression in suitable hosts is being used routinely by major enzyme production companies to produce improved biocatalysts.. Protein engineering is also being used to obtain improved biocatalysts, the case of alkaline protease being a paradigm. Already in the market, thermostable proteases capable to withstand harsh washing conditions (high pH, high concentration of strong oxidants) are products of protein engineering produced by point amino acid substitutions in the most labile region of the molecules In the production of syrups from cornstarch, thermostability of a-amylase is severely reduced below pH 6, which poses the inconvenience of pH adjustment before and after starch liquefaction. A thermostable a-amylase from Bacillus licheniformis, active at low pH and low Ca++concentration has been recently patented. A thermostable glucose isomerase is a major challenge in the production of high-fructose corn syrup. Equilibrium is favored at high temperature, so that at 110 °C 55 % HFCS could be produced at the enzyme reactor stage, without the cumbersome process of sugar fractionation now used. It was shown that specific substitution of a surface arginine residue for lysine, obtained by sitedirected mutagenesis, produced a substantial thermal stabilization in the glucose isomerase from Actinoplanes missouriensis. Protein engineering is a powerful tool for the design of robust biocatalysts and probably most future biocatalysts will be produced by engineered organisms. Genetic gngineering follows the convenience of cloning termophilic genes into more suitable mesophilic hosts. Those systems will be highly productive and the enzymes produced will retain its original thermostability. In fact, in a number of cases thermophilic genes have been cloned and expressed in mesophilic hosts, producing enzymes highly active and stable at high temperatures. Some examples are in Table below and other bacterial hosts pose some problems in expressing genes from archibacteria, because of misreading of intervened genes. E.coli and other bacterial hosts pose some problems in expressing genes from archibacteria, because of misreading of intervened genes. This is not the case with eubacterial genes, being therefore better candidates for cloning into bacterial hosts Following Industrial thermostable enzymes, commercial enzymes from thermophiles and termophilic genes cloned in mesophilic hosts given in the table below. In some cases, remarkable similarities are observed between thermophilic enzymes and their mesophilic counterparts, homology being as high as 85% (Vieille and Zekus, 1996). Thermostability is the result of differences in specific aminoacid sequences and it has been ascribed to a more rigid configuration and to the high number of hydrophobic interactions. By examining primary sequences of termophilic enzymes and mesophilic counterparts the nonconserved regions, as those possibly linked to the thermostable phenotype, can be identified. This opens up the possibility of using protein engineering techniques (Imanaka et al., 1988) to produce point mutations in the mesophilic structural gene, which will result in the corresponding aminoacid substitution in the primary structure of the encoded protein (Daniel, 1996). Good results have been obtained in several cases when replacing aminoacids for those corresponding to the thermophilic protein. Most effective zones for substitution will be the more flexible for being the more labile (Vieille and Zekus, 1996). However, homology between mesophilic enzymes and their thermophilic counterparts are usually between 30 and 50 % and no general strategy for converting mesophilic into thermophilic enzymes have emerged yet, making thermophiles or the genes derived from them the preferred source for thermostable enzymes in the foreseeable future (Adams and Kelly, 1998)