Supplemental Digital Content 1. Supplementary Materials and
advertisement

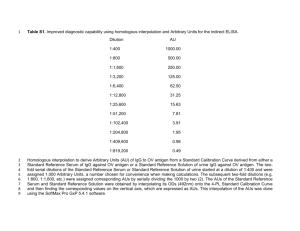
Supplemental Digital Content 1. Supplementary Materials and Methods Enzyme-Linked Immunosorbent Assay Quantification of Anti-PcrV Titers. Micro-well plates (Nunc C96 Maxisorp, Thermo Fisher Scientific Inc., Waltham, MA, USA) were coated for 2 h at 4°C with rePcrV suspended in coating buffer (1.5 μg/mL in coating solution, 0.05M NaHCO3, pH 9.6). Plates were washed twice with phosphate-buffered saline (PBS) with 0.05% Tween-20 (P9416, SigmaAldrich Co., St. Louis, MO, USA) and blocked with 200 μL of 1% bovine serum albumin/PBS overnight at 4°C. Samples (serial dilution: 32–1024×) were applied to the plates (100 μL/well) and incubated overnight at 4°C. Peroxidase-labeled anti-rabbit IgG (A6154, Sigma-Aldrich Co.) or peroxidase-labeled anti-human IgG (A8667, Sigma-Aldrich Co.) were added at dilutions of 1:60,000 for 1 h at 37°C. Then, after six washes, the plates were incubated with 2,2′-azino-bis (3ethylbenzthiazoline-6-sulfonic acid) (A3219, Sigma-Aldrich Co.) at room temperature for 30 min. Next, after the addition of 0.5M H2SO4 (100 μL/well) to the plate, the optical density (OD) at 450 nm was measured by a microplate reader (MTP-880Lab, Corona Electric Co., Hitachinaka, Japan), and dilution indices with OD 450 nm > 0.15 were considered positive. P. aeruginosa Strains The P. aeruginosa strains PA103, PAR001, and PAR004 were used in this study. The PA103 strain was isolated originally from a patient in Australia in the late 1960s, and has a cytotoxic phenotype and the TTSS genotype exoS-, exoT+, exoU+, and exoY+, but has an ExoY secretion-negative phenotype caused by a codon mutation (39-41). The PAR001 strain was isolated from the tracheal aspirate of a 76-year-old patient in 2009, while PAR004 was isolated from the urine of a 15year-old in-patient at the Kyoto Prefectural University of Medicine Hospital. Both strains showed a cytotoxic phenotype, a TTSS genotype (exoS- and exoU+), and are resistant to multiple antimicrobial agents (carbapenems, aminoglycosides, and fluoroquinolones). Note that both PAR001 and PAR004 are TTS-genotype exoS-/exoU+ cytotoxic phenotypes and the lethal doses of PAR001 and PAR004 are 7–10 times as large as that of PA103, suggesting that they are less virulent than PA103. Lung Edema, Bacteriology, White Blood Cells, Mediators, and Histological Measurements. The lung edema index was obtained by measuring the wet to dry weight ratios of the mouse lungs at the 24-h time point as follows: lung homogenates were placed in pre-weighed aluminum pans and dried to a constant weight in an oven at 50°C for 3 days. The lung homogenates were diluted sequentially and plated on sheep blood agar plates for quantitative assessment of the bacterial counts. In a separate experimental setting, mice infected intratracheally with P. aeruginosa and intravenously treated with various therapeutic agents were sacrificed at the 12-h time point after collecting their arterial blood. IL-1β, IL-6 and TNF-α concentrations in lung homogenates and in plasma were quantified by an ELISA kit (BD OptEIA ELISA Sets, BD Biosciences, San Jose, CA, USA). The myeloperoxidase activity of the lung homogenates was measured using a biochemical method reported previously (theLabRats: Protocol for assay of myeloperoxidase in frozen lung tissue in the LabRat.com. 2005 [cited Available from: http://www.thelabrat.com/index.shtml]). The number of white blood cells in the blood was counted by a hemacytometer. For fixation, lungs were perfused with 10% buffered formalin phosphate and embedded in paraffin. Mounted sections were stained with hematoxylin-eosin and observed under a light microscope. Interventions The experiments were performed in three different settings (pre-mixed, pre-iv, and post-iv). In the pre-mixed setting, anti-PcrV polyclonal IgG, IVIG, or albumin was pre-mixed with P. aeruginosa PA103 (1.5 × 106 CFU, 60 µL), and the mixtures were intratracheally administered to the lungs. One group of mice intratracheally received PA103 (1.5 × 106 CFU, 60 µL) solution alone. For the prophylactic intravenous administration experiments before infection (pre-iv setting), either anti-PcrV IgG, IVIG or albumin in 200 µL was intravenously injected 1 hour before tracheal instillation of PA103 (1.5 × 106 CFU, 60 µL). In a separate experiment, either IVIG or albumin was intravenously injected 1 hour before tracheal instillation of either PAR001 or PAR004 (1.0 × 107 CFU, 60 µL). In the therapeutic intravenous administration experiment before infection (post-iv setting) and 3 hours after tracheal instillation of P. aeruginosa (1.5 × 106 CFU, 60 µL), either anti-PcrV IgG, IVIG, ceftazidime (C1635, LKT Laboratories, Inc., St. Paul, MN, USA; CAZ), CAZ plus anti-PcrV IgG, CAZ plus IVIG or albumin solution alone (200 μL) was intravenously administered. In the experiments with specific titer-depleted IgG or IVIG, either anti-PcrV IgG (αPcrV), PcrV-titer depleted anti-PcrV IgG (αPcrV-dep-PcrV), IVIG depleted with rePcrV (IVIG-dep- PcrV), formaldehyde-fixed P. aeruginosa (IVIG-dep-PA), or IVIG depleted with PA and rePcrV (IVIG-dep-PA&PcrV) was mixed with P. aeruginosa (1.5 × 106 CFU, 60 µL), and subsequently instilled intratracheally into the lungs. Supplemental Figure 1. Bactericidal activity tests. To examine the direct bactericidal activity of IVIG, Pseudomonas aeruginosa PA103 (7.5 × 105 CFU/750 µL/well) was incubated in phosphate-buffered saline (PBS) with or without albumins, anti-PcrV IgG (5 µg/well), immunoglobulins (50 µg/well) or ceftazidime (6 or 12 µg/mL) and with or without 10% murine serum (non-heat inactivated) at 37°C. Every hour for 4 hours, the solutions were diluted and plated on sheep blood agar plates for quantitative assessment of the bacterial counts. PBS: phosphate-buffered saline; αPcrV: polyclonal anti-PcrV IgG; Ig: immunoglobulins; Alb: bovine serum albumin; CAZ-6: ceftazidime 6 µg/mL; CAZ12: ceftazidime 12 µg/mL. Data are shown as the mean + SD from triplicate assays (Note: We tested the addition of 10% heat-inactivated serum as a substitute for 10% fresh serum, but found no difference between the fresh and heat-inactivated versions). *, p < 0.05 compared with the PBS-treated or serumtreated; †, p < 0.05, versus the PBS group (left) or serum-group (right). Supplemental Figure 2. Anti-PcrV titers of immunoglobulins at various dilutions. αPcrV: polyclonal anti-PcrV IgG; αPcrV-dep-PcrV: polyclonal anti-PcrV IgG depleted with recombinant PcrV; Ig: immunoglobulins; Ig-dep-PcrV: immunoglobulins depleted with recombinant PcrV; Ig-dep-PA103: immunoglobulins depleted with formaldehyde-fixed Pseudomonas aeruginosa PA103; Ig-dep-PA and PcrV: immunoglobulins depleted with recombinant PcrV and formaldehyde-fixed P. aeruginosa PA103; BSA: bovine serum albumin. †, p<0.05 versus each αPcrV; *, p<0.05 versus each Ig; N.S. = not significant. Data are presented as the mean + SD from triplicate assays. Dilution factors at optical densities (OD, measured at 450 nm) >0.15 were considered as positive titers. Supplemental Figure 3. Lung histology 10 hours post-tracheal instillation of Pseudomonas aeruginosa PA103, PAR001, or PAR004. PAR001 and PAR004 are clinical isolates of P. aeruginosa. In the pre-iv setting, either IVIG or albumin was intravenously administered 1 hour prior to intratracheal instillation of a lethal dose of P. aeruginosa. Ig: immunoglobulin-treated; Alb: bovine serum albumintreated. Lungs were fixed 10 hours after bacterial instillation. Hematoxylin-eosin staining after 10% formaldehyde fixation and paraffin embedding. 200× magnification. Supplemental Figure 4. Lung histology 10 hours post-tracheal instillation of a lethal dose of Pseudomonas aeruginosa. In the pre-mixed setting, a lethal dose of P. aeruginosa PA103 pre-mixed with either the following various IgGs or albumin was instilled intratracheally into the lungs. Ig: immunoglobulin-treated; Alb: bovine serum albumin-treated; Ig-dep-PcrV: immunoglobulins depleted with recombinant PcrV; Ig-dep-PA103: immunoglobulins depleted with formaldehyde-fixed PA103; Ig-dep-PA&PcrV: immunoglobulins depleted with recombinant PcrV and formaldehyde-fixed PA103. Lungs were fixed 10 hours after bacterial instillation. Hematoxylin-eosin staining after 10% formaldehyde fixation and paraffin embedding. 200× magnification.