AAs as ligands and pdb LO_v2
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
Proteins are polymers of amino acids joined together through the formation of peptide bonds (amide linkages). While the oxygen and nitrogen atoms of the peptide bonds may serve as a donor atom to complex a metal ion, very often the ligands for the metal come from the amino acid side chains. In this exercise, we will explore which of the 20 standard amino acids have the potential to serve as ligands in a metalloprotein by viewing some examples found in protein structures on the Protein
Data Bank (PDB).
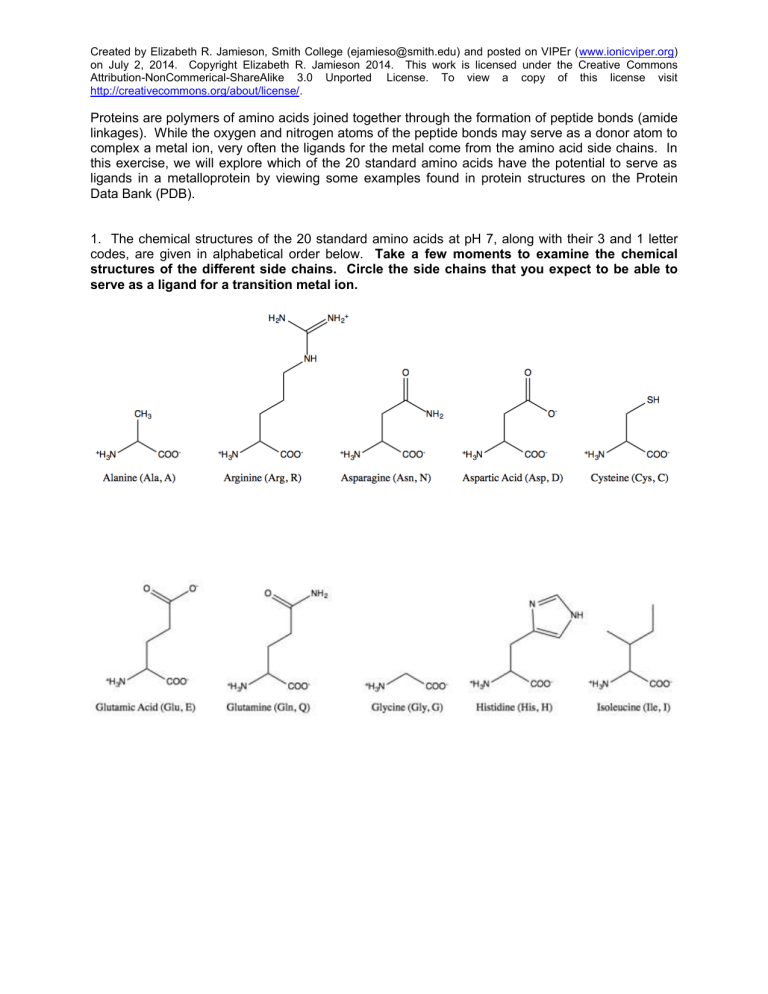
1. The chemical structures of the 20 standard amino acids at pH 7, along with their 3 and 1 letter codes, are given in alphabetical order below. Take a few moments to examine the chemical structures of the different side chains. Circle the side chains that you expect to be able to serve as a ligand for a transition metal ion.
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
2. The Protein Data Bank (PDB, www.pdb.org
) is an online repository of structural information for biomolecules obtained by X-ray crystallography, NMR methods, and cryo-electron microscopy. It has many useful viewing tools to examine the structure of metalloproteins.
In order to use the PDB website to its full potential, you will need a modern web browser (Firefox 4+,
Chrome 10+, Safari 3+) with JavaScript and cookies enabled. Some of the molecular viewing programs require Java 1.7 or above. If you encounter technical difficulties while trying to do the exercise below with the program Ligand Explorer, please consult the PDB’s troubleshooting Java
Web Start problems website at: http://www.pdb.org/pdb/staticHelp.do?p=Viewers/webstart_troubleshooting.jsp
Note: Additional help topics are available by selecting “Launch Help System” in the Help box on the left hand side of the PDB homepage (you may have to scroll down to see the Help box on the left side). The Help box also has a series of video tutorials for using the PDB.
3. Go to the PDB home page ( www.pdb.org
). Each structure on the PDB is given its own PDB ID number. We are going to explore the structure of some metalloproteins using the PDB and a program called Ligand Explorer to see how proteins can act as ligands for transition metal ions.
The first protein that we will examine is called plastocyanin. Its PDB ID number is 1IUZ. Enter the code 1IUZ in the search box at the top of the page and hit return . You will be brought to the
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
structure summary page for plastocyanin that provides the literature reference and other summary information about the molecule. Note the blue tabs at the top of the page above the word
Plastocyanin (such as 3D View, sequence, etc.). You can click on these tabs to obtain more information about the molecule and how the structure was determined.
From the structure summary page, scroll down to the central box labeled, “Ligand Chemical
Component.” You will see Cu (Cu(II) ion) as one of the ligands. Click on the “Ligand Explorer” box to launch the Ligand Explorer program.
You may be asked to open it with Java Web Start – as well as to approve running/installing something that was downloaded from the Internet.
Basic Instructions on how to use Ligand Explorer can be found at: http://www.pdb.org/pdb/staticHelp.do?p=help/viewers/ligandExplorer_viewer.html
Once Ligand Explorer has launched, you can click on the “Metal Interaction” box to show the parts of the protein that are bonded to the Cu(II) ion. Click in the structure window and move your mouse up, down, left and right to rotate the molecule. You can zoom in and out by holding down the shift key and moving your mouse up and down.
Ligand Explorer labels amino acids using the 3 letter codes plus the number where that amino acid occurs in the primary sequence of the protein chain.
In the box at the top of the screen, Ligand Explorer shows the one-letter amino acid codes for the protein chain. The amino acids that are involved in binding the metal ion are highlighted, emphasizing how in proteins, amino acids can be far apart in the linear primary sequence, yet close together in space once protein folding has occurred.
What is the coordination number of the Cu(II) ion and how would you describe its geometry?
________________________________________________________________________________
What amino acid side chains are serving as ligands?
__________________________________
________________________________________________________________________________
Describe (with words or a figure) how the atoms in the side chain coordinate to the Cu(II)
(monodentate, bidentate, bridging ligands, etc.).
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
4. Next we’ll examine Cu/Zn Superoxide Dismutase. Its PDB ID is 2SOD. You can either go back to the PDB homepage and search for 2SOD as we did in part 3 above, or you can use the File:Open
PDB ID menu in Ligand Explorer. Open the 2SOD file in Ligand Explorer .
We are going to examine the Cu and Zn ions in the B chain of the molecule. Under the “choose a ligand to analyze” box, click on CU (B 152) under the B chain. Click on the “Metal Interaction” box.
What is the coordination number of the Cu(II) ion and how would you describe its geometry?
________________________________________________________________________________
What amino acid side chains are serving as ligands?
__________________________________
________________________________________________________________________________
Describe (with words or a figure) how the atoms in the side chain coordinate to the Cu
(monodentate, bidentate, bridging ligands, etc.).
Now , let’s explore the coordination of the Zn ion. Under the “choose a ligand to analyze” box, click on Zn (B153) under the B chain.
What is the coordination number of the Zn(II) ion and how would you describe its geometry?
________________________________________________________________________________
What amino acid side chains are serving as ligands?
__________________________________
________________________________________________________________________________
Describe (with words or a figure) how the atoms in the side chain coordinate to the Zn
(monodentate, bidentate, bridging ligands, etc.).
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
5. The table below lists several other proteins and PDB ID codes selected to give you a full picture of the coordination modes of amino acids. Open each protein in Ligand Explorer. For each of the indicated metal ions, record the coordination geometry, which amino acids are serving as ligands, and how the atoms are coordinating to the metal (monodentate, bidentate, bridging, etc.) in the worksheet below.
Protein
Purple Acid Phosphatase
Methane Monooxygenase
GAL4 transcription factor p21ras protein)
(GTP hydrolyzing
PDB ID
1KBP
1FYZ
1D66
1JAH
Metal Ions to Examine
Chain A FE (A 438) (Fe 3+ )
Chain A ZN (A 439) (Zn 2+ )
Chain B FE2(B 5003) (Fe 2+ )
Chain B FE2 (B 5004) (Fe 2+ )
Chain A CD (A 67) (Cd 2+ )
Chain A CD (A 68) (Cd 2+ )
Chain A MG (A168) (Mg 2+ )
(Note: also turn on “Neighbor
R esidues” to see complete metal coordination)
Metal Ion Coordination
Number/Geometry
Amino Acid Ligands Description of
Coordination
Purple Acid
Phosphatase
FE (A 438)
Purple Acid
Phosphatase
ZN (A 439)
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
Metal Ion Coordination
Number/Geometry
Amino Acid Ligands Description of
Coordination
Methane
Monooxygenase
FE2(B 5003)
Methane
Monooxygenase
FE2(B 5004)
GAL4 transcription factor
CD (A 67)
GAL4 transcription factor
CD (A 68)
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
Metal Ion Coordination
Number/Geometry
Amino Acid Ligands Description of
Coordination p21ras
MG (A168)
6. Now that you’ve had a chance to explore the metal ion coordination in several proteins, go back to the amino acids you circled in part 1 as likely to bind to a metal ion. How well did your predictions match what you observed in the protein structures on the PDB?
C ompare your results to what’s described in Bertini, Gray, Steifel, and Valentine’s Biological
Inorganic Chemistry: Structure & Reactivity, Chapter III (focusing on section III.3 and Figure
III.2). Were you able to find examples of all the coordination modes they describe?
7. Hard Soft Acid Base (HSAB) theory is often invoked to explain ligand specificity when metal ions bind to proteins. Go back and re-examine the ligand-metal relationships you observed in parts
3-5 above. Based on what you found, how well does HSAB theory hold in predicting which amino acid side chains are likely to bind to particular metal ions?
Created by Elizabeth R. Jamieson, Smith College (ejamieso@smith.edu) and posted on VIPEr ( www.ionicviper.org
) on July 2, 2014. Copyright Elizabeth R. Jamieson 2014. This work is licensed under the Creative Commons
Attribution-NonCommerical-ShareAlike 3.0 Unported License. To view a copy of this license visit http://creativecommons.org/about/license/ .
8. One way that nature can provide greater ligand variety than the standard 20 amino acid side chains is through the use of post-translational modifications. A post-translational modification is when an amino acid side chain is covalently modified after the protein chain has been synthesized by the ribozyme. One common example of post-translational modification is the addition/deletion of a phosphate group to the amino acid serine to control an enzyme ’s activity. A particular enzyme may become more active when a regulatory kinase enzyme phosphorylates it at a specific serine, and its activity will decrease when a regulatory phosphatase enzyme removes this group.
In terms of post-translational modifications making a difference for metal binding, one great example is the post-translational modification that occurs in the protein prothrombin (important for blood clotting). Take a look at prothrombin (PDB ID 2PF2) and describe the modification to the amino acid side chains in the Ca 2+ binding sites. Explain how this modification would make this protein better able to bind Ca 2+ .
Another example of post-translational modifications adding variety for metal binding can be found in the enzyme nitrile hydratase where the side chains of cysteine residues are converted into sulfenate and sulfinate ligands. If you would like to examine the structure of a cobalt nitrile hydratase, its PDB
ID is 1UGP. You will note that in addition to the cysteine and modified cysteine residues, other coordinating atoms come from the amide nitrogens in the protein backbone.
9. Finally, g o to the PDB’s home page at www.pdb.org
. You will notice in the center of the webpage a fun and informative feature called, “Molecule of the Month.” The PDB features a different molecule each month and provides a short summary on its function and structure. If you click on the name of the molecule (in red) or the words full article (also in red), you will be able to access the write-up for this month’s featured molecule. Once in the article, if you click on the tab at the top labeled,
“Molecule of the Month,” you will be brought to the archive page for this feature. Take a scroll down the page. Do you recognize any interesting metalloproteins on this list? If so, take a moment to explore the coordination environment of the metal and briefly describe it below.






