Hematologic Problems
advertisement

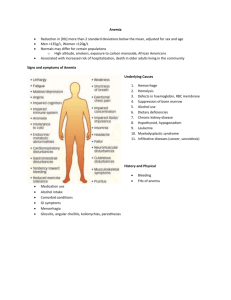
NUR 475 – FNP III Hematologic Problems Interpreting an Abnormal Complete Blood Cell Count in Adults Variables to focus on when examining the CBC: Hgb (as general indicator of anemia or polycythemia) MCV (useful in classifying anemias) (Normal 86-98) (MCH 28-35) RBC distribution width (helpful in differential diagnosis of anemia) o Normal: 11.5-14.5% o Calculated from the MCV and the RBC count o Quantitative measure of anisocytosis (RBCs unequal in size) o May become abnormal before the anemia occurs RBC count (an increased RBC count associated with anemia is characteristic in thalassemia trait) Platelet count (to detect either thrombocytopenia or thrombocythemia) WBC count with differential (usually gives important clues for diagnosis of acute leukemia and chronic lymphoid or myeloid diseases as well as for the presence of leucopenia and neutropenia) o NOTE: In patients with an abnormal WBC count, immediately ask which WBC type is affected: neutrophils, lymphocytes, monocytes, eosinophils,or basophils What is “abnormal?” Interpret an “abnormal” CBC within the context of an individual’s baseline value o Up to 5% of the general population without disease may display laboratory values outside the statistically assigned “normal” reference range o Always review old medical records before initiating a costly work-up of an “abnormal” CBC. An individual may display a substantial change from his or her baseline (personal normal) and still fall within the “normal” reference range Consider differences in the CBC based on race and sex o RBC-associated measurements are lower and platelet counts are higher in women than men o Persons of African ancestry display significantly lower Hgb, WBC, neutrophil, and platelet counts than white persons History & Physical Patient history: age, sex, race, diet, infection, inheritance, diarrhea (suspect small bowel disease with malabsorption of folate or B12, suspect blood loss) Family history: anemia, jaundice, splenomegaly, bleeding tendencies, drug/toxin exposures, bone marrow failure, chronic disease Patient’s nutritional history: o Dietary intake of iron sources, vitamins, milk, meat, vegetables o 24 hour dietary recall o History of pica (an eating disorder typically defined as the persistent eating of nonnutritive substances for a period of at least 1 month at an age in which this behavior is developmentally inappropriate (e.g., > 18-24 mo). The substances include such things as clay, dirt, sand, stones, pebbles, hair, lead, plastic, ice, paper, paint chips, coal, chalk, wood, and plaster.) Review of systems: jaundice, extremity pain, abdominal pain, blood loss, weight loss, recent infections, drug exposures, travel, behavioral changes, pallor, petechiae, ecchymoses, adenopathy, GI/GU disorders, changes in stool indicating GI bleeding Physical Examination: o Skin: pallor, jaundice, excessive bruising, petechiae o HEENT: fundal hemorrhages, mucous membrane bleeding, lymphadenopathy, frontal bossing o CV: heart murmurs, heart failure o Abdominal: hepatosplenomegaly o MSK: joint or extremity pain Anemia Definition: reduction in the red cell mass or the blood hemoglobin concentration; hemoglobin level less than the lower range value for age First step: classify the process to narrow the differential diagnoses that need to be considered o Microcytic (MCV < 80 fL) o Normocytic (MCV 80-100 fL) o Macrocytic (MCV > 100 fL) Obtain a peripheral blood smear (PBS) during the initial evaluation of anemia o Enhances the initial process of differential diagnosis and provides guidance for further testing Microcytic Anemia (= small cells) - ↓ MCV 3 major diagnostic possibilities: o Iron deficiency anemia (most common) Increased RDW Thrombocytosis o Thalassemia Normal or elevated RBC count Normal or elevated RDW o Anemia of chronic disease/inflammation Normal RDW 3 stages of iron deficiency anemia o Stage 1: ferritin and hemosiderin stores depleted o Stage 2: serum iron deceaesed, iron binding capacity increased o Stage 3: Hemoglobin decreased, iron deficiency affects heme synthesis Check serum ferritin level o If low: diagnostic of iron deficiency anemia o If normal: determine whether the microcytosis is new If chronic, consider diagnosis of thalassemia: order Hgb electrophoresis Normal in α-thalassemia trait Abnormal in β-thalassemia trait Hematology consult may be necessary for accurate diagnosis If acquired (new) microcytosis and not iron deficiency (with normal or elevated ferritin level), indicative of underlying systemic disease. Labeled as microcytic anemia of chronic disease Usual causes: temporal arteritis, rheumatoid arthritis, chronic inflammation, chronic infection Unusual causes: Hodgkin lymphoma, renal cell carcinoma, Castleman disease (noncancerous growths in lymph tissue), myelofibrosis Normocytic Anemia (= normal sized cells) First: rule out treatable causes o bleeding (CBC usually unremarkable) check patient history fecal occult blood test if indicated o nutritional anemia (increased RDW) both iron and vitamin B12/folate deficiencies are possible causes of normocytic anemia (“average out”) o anemia of renal insufficiency (normal RDW) decreased erythropoietin can check serum creatinine o anemia of chronic disease/inflammation o concomitant low MCV and high MCV conditions o hemolysis (normal or elevated RDW, thrombocytosis) always consider possibility of drug-induced hemolysis Macrocytic Anemia (=Large cells) First consideration: use of certain drugs (hydroxyurea, zidovudine), alcohol abuse Rule out nutritional causes (B12 or folate deficiency) o Check homocysteine and B12 levels If both are normal: B12 or folate deficiency unlikely If MCV 100-110 Consider myelodysplastic syndrome as well as liver disease, alcohol consumption, hypothyroidism, and marked reticulocytosis from hemolysis If MCV > 110 Consider myelodysplastic syndrome or other primary bone marrow disorder Marked macrocytosis that is not secondary to either nutritional deficiency or drug effect almost always is associated with a primary bone marrow disease (e.g. myelodysplastic syndrome, aplastic anemia, pure red cell aplasia, or large granular lymphocyte disease). If a specific hematologic diagnosis will affect management decisions, obtain bone marrow biopsy. (CBC = complete blood count; RBC = red blood cell; MCV = mean corpuscular volume; TIBC = total iron-binding capacity; MMA = methylmalonic acid) Algorithm for evaluation of anemia in the elderly (Reference: Smith, D.L. (Oct 1, 2000). Anemia in the elderly. Am Fam Physician, 62(7), 1565-1572.) Iron Deficiency Anemia Risk factors o Prominent in age groups experiencing rates of rapid growth: toddlers, adolescents, and pregnant and lactating women o Black race o Blood donation > 2 units/year in women and 3 units/year in men o Low socioeconomic status and postpartum status o Mexican ethnicity living in the US o Child and adolescent obesity o Vegetarian diet Usual causes o Blood loss o Dietary insufficiency o Decreased absorption due to gastric surgery or heavy antacid use Clinical presentation o Fatigue caused by anemia in only 1 out of 52 patients in primary care o Pallor, spoon nails, glossitis, pale tachycardia, mild systolic ejection murmur Testing: in addition to labs, remember colonoscopy! Treatment o Consider transfusion for patients of any age c/o symptoms such as fatigue or DOE, and for asymptomatic cardiac patients with hemoglobin < 10. o One month trial of oral iron: ferrous sulfate 325 mg tid until Hgb returns to normal, the 3-6 months of bid dosing to replenish ferritin stores. Initiating with once-a-day dosing and gradually titrating upward may help minimize GI intolerance Adding vitamin C potentiates iron absorption Liquid iron stains teeth unless brushed right away; use straw An increase in the Hgb level of 1 g should occur every 2-3 weeks on iron therapy; however, it may take up to 4 months for the iron stores to return to normal after the hemoglobin has corrected. Reassess Hgb after 3-4 weeks to document improvement Therapy should continue with bid dosing Thalassemia Abnormal Hgb produced excessive RBC destruction; small cells (↓ MCV) o Hgb made of 2 proteins: α-globin and β-globin α-thalassemia – southeast Asia, Middle East, china, African descent β-thalassemia (Cooley’s anemia) – Mediterranean, Chinese, other Asian, African American Thalassemia major – homozygous; symptomatic, require treatment o Treatment: blood transfusions, folate supplements Caution: Watch for iron overload with blood transfusions o Without treatment, severe thalassemia major leads to heart failure and early death (age 20-30) Thalassemia minor – heterozygous; “silent carrier”, no symptoms Anemia of Chronic Disease (ACD) Anemia often accompanies chronic inflammation or malignancy due to: o Decreased RBC longevity o Decreased erythropoiesis May become microcytic over time Rarely causes drop of Hgb < 9 unless accompanied by coexisting pathology Iron deficiency anemia frequently coexists with ACD Labs: o Like iron deficiency: serum iron and transferrin saturation low o Unlike iron deficiency: ferritin is normal or increased or TIBC is low Causes o Autoimmune disease o Chronic infection (TB, HIV, endocarditis) o Malignancy o Liver disease o Chronic renal failure Treatment o Aimed at underlying disease process o If Hgb < 9, look for coexisting pathology (B12 or iron deficiency, multiple myeloma) o If renal failure: erythropoietin to no more than 12 Hgb, vitamin C, E, iron o Empiric trial of iron to treat any coexistent iron deficiency Vitamin B12 Deficiency Causes, especially among the elderly o Inadequate ingestion (chronic alcoholism, chronic malnutrition, strict vegetarianism with no consumption of animal or dairy products) o Inadequate absorption (atrophic gastritis, gastrectomy, intestinal resection, malabsorption, Crohn’s disease, chronic pancreatitis) Pernicious anemia = a chronic, progressive, macrocytic anemia caused by a deficiency in intrinsic factor (substance necessary for B12 absorption in the GI tract; autoimmune diseae most common in women age 35-60 of Northern or Eastern European ancestry o Inadequate stores (advanced liver disease) o Drug-induced causes of B12 deficiency: metformin, acid-suppressive therapy (PPIs, H2 receptor blockers, antacids) Mechanism: thought to be inhibition of calcium-dependent ileal absorption of the B12-intrinsic factor complex, which calcium supplementation can reverse (Bauman, et al, 2000) Clinical manifestations o HEENT: glossitis, angular cheilitis o GI: anorexia, diarrhea o Neurologic: paresthesias, peripheral neuropathy, difficulty with balance, positive Romberg, o Psychiatric: irritability, personality change, mild memory impairment, dementia, depression, psychosis o Cardiovascular: possible increased risk of MI and stroke Treatment: Vitamin B12 replacement therapy (Note: Although the majority of dietary vitamin B12 is absorbed in the terminal ileum through a complex with intrinsic factor, evidence for an alternate transport system is mounting.) o o o o o o Oral: Initial dose: 1,000-2,000 mcg/day for 1-2 weeks; Maintenance dose: 1,000 mcg/day for life IM: Initial dose: 100-1,000 mcg every day or every other day for 1-2 weeks; Maintenance dose: 100-1,000 mcg every 1-3 months Sublingual: 2,000 mcg/d Intranasal: 500 mcg weekly May need to supplement folate and iron stores as well (concomitant deficiencies) First few days of treatment: The older adult is at risk for heart failure and hypokalemia. Why? Folate Deficiency At high risk o Persons with poor dietary intake o Persons with rapid growth such as adolescents and pregnant women o More common in alcoholic and chronically malnourished persons o Low levels of folate can disrupt transmethylation reaction, leading to an accumulation of homocysteine Medications which can interfere with folate utilization: anticonvulsants, metformin, methotrexate, sulfasalazine, triamterene, oral contraceptives Clinical manifestations: glossitis, angular cheilitis, anorexia, diarrhea, pale or icteric skin, fine brittle hair, pica Treatment: Replace with folic acid 1 mg/d p.o. o Recheck folate levels in 2 months to determine if correct amount of folate is being used o Encourage dietary intake Spinach, liver, yeast, asparagus, and Brussels sprouts are among the foods with the highest levels of folate. Other sources: vegetables (especially dark green leafy vegetables), fruits and fruit juices, nuts, beans, peas, dairy products, poultry and meat, eggs, seafood, and grains o B12 should always be assessed in presence of folate deficiency Folate replacement will mask hematologic findings but not prevent neurological sequelae caused by coexistent B12 deficiency Anemia in the Elderly Read: “Anemia in Older Persons” at http://www.aafp.org/afp/2010/0901/p480.pdf Associated with poor clinical outcomes and increased mortality o Negative outcomes include: falls, fatigue, frailty, functional decline, decreased cognition, impaired mobility, MI, restricted mobility, increased hospitalizations, and impaired quality of life. o Hypoxia from anemia increases peripheral arterial vasodilation, myocardial dysfunction, and activation of the sympathetic and rennin-angiotensin system to maintain BP; these changes could result in the onset or progression of heart and renal failure. o Patients with heart failure whose hemoglobin measurements are in the lowest quartile have more symptoms, poorer hemodynamics, and greater mortality than those with higher hemoglobin levels. Prevalence of > 10% (higher prevalence in long-term care 30-50%) Causes of anemia in the elderly o Nutritional anemia (1/3 of anemia cases) Iron deficiency, including chronic blood loss Folate deficiency (related to excessive alcohol use and malnutrition) Vitamin B12 deficiency (primarily related to atrophic gastritis) > 10% of elderly have borderline or low vitamin B12 levels o Anemia of renal insufficiency or chronic inflammation o Myelodysplastic syndrome Primary disorder of hematopoiesis Median age of onset = seventh decade of life Signs/symptoms: Nonspecific o Fatigue, headache, dyspnea, dizziness, syncope, depression, decreased thought processes, tachycardia, palpitations, cold intolerance, edema, systolic ejection murmur, orthostasis, anorexia, impotence, pale skin, mucous membranes and conjunctiva Another classification: Disorders of RBC production (NOTE: if all 3 [RBC, WBC, Platelets] are low, think leukemia) o Aplastic anemia o Anemia secondary to malignancies and/or chronic disease Disorders of RBC maturation o Iron deficiency o Lead poisoning (especially in homes built before 1960s, occupations with lead) o Thalassemia syndromes Disorders of RBC destruction o Sickle cell anemia o G6PD deficiency o Pyruvate kinase deficiency o Hereditary spherocytosis Myelodysplastic syndromes (MDSs) Definition: a group of bone marrow disorders that lead to underproduction of normal blood cells. Multilineage cytopenia (low RBC, WBC, and platelets) is not simply a normal consequence of aging; it warrants referral to a hematologist-oncologist. o Diagnosis of MDS implies a risk of developing acute myelogenous leukemia (AML) that is much greater than the expected rate for a healthy, age-matched control population. 20-30% of patients with MDS develop AML AML arising in the setting of MDS is almost always lethal MDS subtypes often categorized as lower-risk or higher-risk, depending on the likelihood of transforming to AML Lower-risk MDS survive a median of 3-7 years Higher-risk types either develop AML or die of complications of MDS, on average within 1.5 years. o Other fatal complications of MDS-associated cytopenias Infections due to neutropenia are leading cause of death due to MDS, killing 4065% of affected patients Life-threatening bleeding due to thrombocytopenia Incidence o Incidence rate increased from 3.28 per 100,00 per year in 2001 to 3.56 per 100,000 per year in 2004 Translate to 10,000-15,000 new cases per year Increase attributed to enhanced awareness of the disease and to the aging of the population o Most MDS patients are older (median age at diagnosis is 71, with 72% of patients age 70 or older; prevalence increases with age In East Asia, occurs almost 2 decades younger than in the rest of the world. More common in men than women; in whites than blacks o Causes o Cause of primary MDS is not known o Smoking increases the risk of MDS. o Genetic and environmental factors probably both play a role Congenital conditions such as Down syndrome, Fanconi anemia, and Bloom syndrome are associated with MDS MDS rarely runs in families o About 10% of cases of MDS are secondary, usually due to radiation treatment or chemotherapy for cancer (time from treatment to the development of MDS is about 5 years). When to suspect MDS o An asymptomatic individual may be found to have blood and bone marrow features that are typical of MDS during a medical evaluation performed for another reason. o Patients may present with signs or symptoms suggestive of a blood disorder: fatigue, exercise intolerance, pallor, frequent infection, or inappropriate bleeding and bruising. These symptoms develop as the bone marrow’s ability to produce normalfunctioning blood cells is more and more compromised. The range of symptoms depends on the bone marrow cell type affected. Laboratory testing/Diagnostic considerations o About 80% of patients with MDS are anemic at presentation Can be microcytic, normocytic, or, most commonly, macrocytic o Can do usual testing for anemia: iron, vitamin B12, and folate levels¸ fecal occult blood testing, liver, renal, and thyroid function tests, o 3 cardinal MDS-associated quantitative hematologic features Erythrocyte macrocytosis Monocytosis Cytopenias in any lineage o Don’t forget to check medications for one which can cause megaloblastoid erythropoiesis Methotrexate, valproic acid (Depakote), phenytoin (Dilantin), Phenobarbital, sulfasalazine, and zidovudine (Retrovir) o Assess for responsiveness of bone marrow to anemia via a reticulocyte count and/or an erythropoietin level (done before any blood transfusion) o Screen for relevant infections, including IV, hepatitis, or in rare cases, parvovirus o Screen for lifestyle factors that may result in bone marrow suppression, such as excessive alcohol intake o Multilineage cytopenia (low RBC, WBC, and platelets) is not simply a normal consequence of aging; it warrants referral to a hematologist-oncologist, especially if above tests are negative. Supportive care o Transfusions: almost all MDS patients need RBCs; fewer need platelets o Iron chelation: can get iron overload from multiple transfusions; this intervention should be reserved for patients with lower-risk disease who are expected to survive > 1 year and who have received > 25 units of packed RBCs. o Antibiotics for those with neutropenia and fever > 100.4o F: need to be hospitalized and given broad-spectrum antibiotics Other treatments o For lower-risk disease: These patients can continue follow-up with their primary care provider once treatment goals and plans are established. Treatment: erythropoiesis-stimulating agents (ESAs) such as Procrit and Arnaesp. About 40% of patients ultimately respond to an ESA, but those who respond eventually develop resistance to the agent. The recommended threshold hemoglobin level for starting an ESA is< 10 g/dL. The agent should be stopped once the hemoglobin level reaches 12 g/dL If ESA treatment is ineffective, immunosuppressive therapy may be used. o For higher-risk disease: should be followed by a hematologist or medical oncologist; can try chemotherapy o Hematopoietic stem cell transplantation is the only curative treatment for MDS. However it is performed in fewer than 5% of patients, usually younger patients with few comorbidities, because the rate of transplant-related death is high. Hemochromotosis Definition: too much iron in the body (iron overload); over time this extra iron may accumulate in various organs, including the liver, pancreas, and heart, causing tissue damage and dysfunction. 2 types o Primary: genetic disorder; absorb too much iron through the GI tract (normal= 10%, with this type of hemochromatosis = 30% absorption) NOTE: the manifestations and complications of this form are fully preventable if the disease is recognized early enough and adequately treated. But, by the time a patient presents with symptoms or is referred for care, the disease phenotype may be well-established. o Secondary: acquired; due to: other blood-related disorders such as thalassemia or certain anemias from having received many blood transfusions long-term alcoholism Incidence: Men > women; prominent in Caucasians of northern European descent; symptoms begin to show between ages 40 and 60 Symptoms: Abdominal pain, fatigue, generalized darkening of skin (bronzing), joint pain, lack of energy, loss of body hair, decreased libido, weight loss, weakness Labs: CBC, ferritin, iron, transferrin saturation (increased), liver biopsy, genetic testing o Transferrin saturation measures the amount of iron bound to protein (transferring) that carries iron in the blood (> 45% is too high) Treatment o Phlebotomy: ½ L/week until iron is within normal limits o If untreated: liver damage (cirrhosis, liver failure, cancer). Can also build up in thyroid, testicles, pancreas, pituitary, heart, joints. Multiple Myeloma (MM) Definition: a progressive hematologic disease characterized by an excessive number of abnormal plasma cells in the bone marrow and by the overproduction of monoclonal immunoglobulins or Bence-Jones protein Incidence: accounts for 1% of all cancers and causes 2% of all cancer deaths, median age of onset is 70 year; somewhat more prevalent in men; more than twice as many cases in African Americans as white Americans. Precise cause is unknown. Clinical manifestations result from the infiltration of plasma cells into the bone marrow and the secretion of MM protein into the blood and urine o New-onset bone pain secondary to severe osteopenia or multiple spinal compression fractures, fatigue, recurrent infections, anemia, hypercalcemia, or renal insufficiency. 70% present with persistent, unexplained bone pain in the lower back, chest, arms, or legs (describe the back pain as aggravated by movement or by lying in a supine position or as a band-like distribution around the body o 60% has normocytic, normochromic anemia Criteria for diagnosis: o Presence of at least 10% abnormal plasma cells in the bone marrow o Monoclonal protein present in the serum or urine o MM-related organ dysfunction (anemia, hypercalcemia, bone lesions or renal insufficiency) What should the NP do o Complete H & P o CBC, CMP, serum protein electrophoresis and immunofixation, 24-hour urine collection for protein, electrophoresis, and immunofixation o Plain skeletal radiography of the spine, pelvis, skull, humeri, and femurs o Refer to hematologist for bone marrow biopsy, etc. Prognosis: survival can vary from months to years Treatment o Autologous stem cell transplant o Chemotherapy Thrombocytopenia Healthy women may experience mild to moderate thrombocytopenia (75,000-150,000) during pregnancy Step 1: make sure not caused by EDTA-induced platelet clumping o Repeat CBC using sodium citrate as anticoagulant Step 2: Consider possibility of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (hematology consultation) Step 3: Consider drug-related thrombocytopenia and hypersplenism o Analgesics (acetaminophen, some NSAIDs) o antibiotics (trimethoprim-sulfamethoxazole, vancomycin) o cardiac meds (amiodarone, digoxin, quinidine, methyldopa, monoxidil, nitroglycerine) o CNS meds (chlorpromazine, diazepam, haloperidol, lithium) o GI meds (cimetidine, sulfasalazine, mesalamie) o thiazide diuretics o antirheumatics (gold salts) o others (atorvastatin, tamoxifin) o heparin Do not miss HIT (heparin-induced thrombocytopenia) Requires immediate cessation of heparin, including heparin flushes For other rare causes (such as post-transfusion purpura), obtain hematology consultation. Leukopenia Neutropenia: increased risk of infection o Most frequent cause: drug therapy (anticonvulsants, thyroid inhibitors, antibiotics, antipsychotics, antidysrhythmics, antirheumatics, NSAIDS) Lymphopenia: due to immunosuppressive drugs, including corticosteroids; viral infections including AIDS Bleeding and Bruising Read: Ballas, M., & Kaut, E.H. (April 15, 2008) Bleeding and bruising: A diagnostic work-up. American Family Physician, 77(8), 1117-1124 at http://www.aafp.org/afp/2008/0415/p1117.pdf Factors that accelerate clot formation: Factor V and VIII accelerate conversion of X --> Xa Factors that inhibit clot formation: Protein C, Protein S, and Thrombomodulin. These form a complete that inactivates VIII and V This complex is activated by thrombin (IIa). Antithrombin blocks XI, IX, X, and II (Prothombin). Fibrinogen: • increased by estrogen, birth control pills, and smoking • Alternatives for female smokers:Norplant,Depo-Provera, progestin Left side (extrinsic pathway) is initiated by damage outside the blood vessel (in the tissue) * factor VII is: - dependent on Vitamin K - inhibited by warfarin (Coumadin) - measured by PT/INR Major increase in intake of Vitamin K-containing foods (green, leafy vegetables) can effect PT/INR (What would be the expected effect?) INR (International Normalized Ratio) • standardizes tests among hospitals, labs, and offices • normal is 2-3 when on Coumadin • Exceptions: normal needs to be higher for those with artificial heart valves (2.5-3.5) Coumadin is very sensitive to drugs (Epocrates lists >300 interactions with prescription, herbal, and over-the-counter medications!): These are due to interactions through the cytochrome P450 system (enzyme induction and enzyme inhibition) and reduced plasma protein binding. Right side of cascade (intrinsic pathway) is initiated by damage within the blood vessel Factor XII named after Dr. John Hageman Factor XI Factor IX (Christmas factor) named after patient (deficiency causes Christmas disease, or Hemophilia type B) Factor VIII (AHF, or antihemophilia factor) (deficiency causes Hemophilia type A) o made in endothelial cells living the blood vessels o It takes 200-2,000 donors to make 1 vial of cryoprecipitate. Prior to heat treatment of cryoprecipitate, there was HIGH risk of HIV. Heparin blocks the intrinsic cascade - monitor with PTT/aPTT When an individual develops a thrombosis, a hypercoagulation panel may be checked. The individual tests included in the panel vary, but at minimum include Factor V Leiden, Prothrombin, Protein C and S, Antithrombin, and homocysteine. Hereditary Risk Factor Prevalence in Caucasian Population Prevalence in Patients with DVT Relative Risk Factor V Leiden 5% 20% 8 Prothrombin 2% 6% 3 Homocysteinemia 5% 10% 2.5 Protein C 0.3% 3% 10 Protein S 0.3% 2% 10 Antithrombin 0.02% 1% 10-50 According to the “second hit” theory for initiation of thrombosis, the presence of more than one risk factor is needed to manifest thrombosis in most patients. For example, 1 hereditary risk factor plus 1 acquired risk factor results in thrombosis. A patient with the factor V Leiden mutation (1st hit) who uses oral contraceptives (2nd hit) greatly increases their risk of thrombosis by combining the 2 risk factors. (Source of above table: http://www.clinlabnavigator.com/hypercoagulable-panel.html) Relative Risk of Venous Thrombosis Thrombophilic Status Normal 1 Oral contraceptive (OCP) use 4 Factor V Leiden, heterozygous 5 to 7 Factor V Leiden, heterozygous + OCP 30 to 35 Factor V Leiden, homozygous 80 Factor V Leiden, homozygous + OCP ??? >100 Prothrombin Gene Mutation, heterozygous 3 Prothrombin Gene Mutation, homozygous ??? possible risk of arterial thrombosis Prothrombin Gene Mutation, heterozygous + OCP 16 Protein C deficiency, heterozygous 7 Protein C deficiency, homozygous Severe thrombosis at birth Protein S deficiency, heterozygous 6 Protein S deficiency, homozygous Severe thrombosis at birth Antithrombin deficiency, heterozygous 5 Antithrombin deficiency, homozygous Thought to be lethal prior to birth Hyperhomocysteinemia 2 to 4 Hyperhomocysteinemia combined with Factor V Leiden, heterozygous 20 *The terms heterozygous (hetero-different) and homozygous (homo-same) are terms used in genetics. The human genome contains to copies of the information. If the copies are the same, they are homozygous; if the copies are different, they are heterozygous. For example, take a protein called A. The normal genome would code for the protein as AA. This is homozygous for the normal protein. If there is a variation of the protein called a, there are two possible ways to get the a. The genome could be Aa, which is called heterozygous or the genome could be aa, which is called homozygous. References: Barzei, A., & Sekeres, M.A. (January 2010). Myelodysplastic syndromes: A practical approach to diagnosis and treatment. Cleveland Clinic Journal of Medicine, 77(1), 37-44. Bauman, W.A., Shaw, S., Jayatilleke, E., Spungen, A.M., & Herbert, V. (September 2000). Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 23:12271231. Dharmarajan, TS,Adiga, GU, & Norkus, EP (March 2003). Vitamin B12 deficiency: Recognizing subtle symptoms in older adults. Geriatrics, 58(3), 30-38. Killip, S. Bennett, JM, & Chambers, MD (March 1, 2007). Iron deficiency anemia. American Family Physician, 75(5), 671-678. Oh, RC, & Brown, DL(March 1, 2003). Vitamin B12 deficiency. American Family Physician, 67(5), 979986. Saint Louis University School of Medicine (2006). Anemia in older persons. Aging Successfully, XVI (2), 1-9. Smith, D.L. (Oct 1, 2000). Anemia in the elderly. Am Fam Physician, 62(7), 1565-1572. Steensma, DP & Tefferi, A. (August 2007). Anemia in the elderly: How should we define it, when does it matter, and what can be done? Mayo Clinic Proceedings, 82(8), 958-966. Tefferi, A, Hanson, CA, & Inwards, DJ (July 2005). How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clinic Proceedings, 80(7), 923-936. Tefferi, A., & Verdiman, J.W. (November 5, 2009). Myelodysplastic syndromes. N. Engl J Med¸361(19), 1872-1885.