Elements and Energy Flow
advertisement

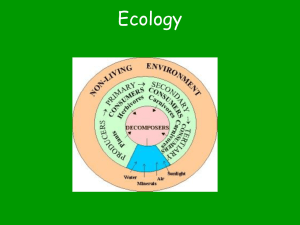
Matter Energy and Ecosystems Chapter 2 After this lecture, you will be able to Describe the nature of environmental systems Explain and apply the fundamentals of environmental chemistry Describe the molecular building blocks of organisms Differentiate among the types of energy and explain the basis of energy flow Distinguish photosynthesis and respiration and summarize their importance to living organisms Define ecosystem and evaluate how living and non-living interact Assess ecosystem services and how they benefit our lives Describe how carbon, phosphorus, nitrogen, and water cycle through the environment Negative feedback loop In a negative feedback loop, the output resulting from a system moving in one direction acts as an input that moves the system in the other direction Input and output neutralize one another Stabilizes the system Most systems in nature involve negative feedback loops Positive feedback loop A positive feedback loop drives it further toward an extreme rather than stabilizing it Runaway cycles of positive feedback are rare in nature but are common in natural systems altered by humans Vanishing Oysters of the Chesapeake Bay Chesapeake Bay was the world’s largest oyster fishery Overharvesting, pollution, and habitat destruction ruined it The economy lost $4 billion from 1980 to 2010 Strict pollution standards and oyster restoration efforts give reason for hope Oyster fishery The Chesapeake Bay Sources of nitrogen and phosphorus Eutrophication in aquatic systems Global hypoxic dead zones Why do energy and chemistry matter? “Every organism is a chemical factory that captures matter and energy from its environment and transforms them into structures and processes that make life possible.” Matter Matter is all material in the universe that has mass and occupies space It can be solid, liquid, or gas Law of conservation of matter Matter can be transformed from one type of substance into others but it cannot be destroyed or created Because the amount of matter stays constant… o It is recycled in nutrient cycles and ecosystems o Pollution and waste don’t go “away” Elements An element is a fundamental type of matter A chemical substance with a given set of properties Nutrients are elements needed in large amounts by organisms Examples: carbon, nitrogen, calcium Molecules and compounds Molecules are combinations of two or more atoms Chemical formula: indicates the type and number of atoms in the molecule (oxygen gas: O2) A compound is a molecule composed of atoms of two or more different elements o Water: two hydrogen atoms bonded to one oxygen atom: H2O Carbon dioxide: one carbon atom with two oxygen atoms: CO2 o Hydrogen ions determine acidity Water can split into H+ and OH– The pH scale quantifies the acidity or basicity of solutions Acidic solutions: pH < 7 Contain more H+ Basic solutions: pH > 7 Contain more OH– Neutral solutions: pH: 7 A pH of 6 contains 10 times as many H+ as a pH of 7 Matter is composed of compounds Living things depend on organic compounds Organic compounds consist of carbon atoms bonded together They may include other elements such as nitrogen, oxygen, sulfur, and phosphorus Carbon can be linked in elaborate chains, rings, other structures Inorganic compounds lack the carbon–carbon bond Hydrocarbons Hydrocarbons are organic compounds that contain only carbon and hydrogen The simplest hydrocarbon is methane (natural gas) Fossil fuels consist of hydrocarbons Crude oil contains hundreds of types of hydrocarbons Organic compounds Material making up biomolecules and macromolecules Formed by rings and chains of carbon Four major categories: Lipids Carbohydrates Proteins Nucleic Acids Cells compartmentalize macromolecules All living things are composed of cells, which are the most basic unit of organismal organization Cells vary in size, shape, and function They are classified according to their structure Cells Cells are surrounded by lipid membrane controlling flow of materials in and out of cell Enzymes are proteins that serve as molecular catalysts, regulating chemical reactions Most chemical reactions require an initial input of energy, or energy of activation, to get started Enzymes, rather than heat, are used in cells to catalyze chemical reactions Energy Energy is the ability to do work Kinetic - Energy in moving objects Potential - Stored energy Chemical - Stored in food or fossil fuels Changing potential into kinetic energy Releases energy Produces motion, action, or heat Potential vs. kinetic energy Potential energy stored in our food becomes kinetic energy when we exercise and releases carbon dioxide, water, and heat as by-products Thermodynamics First Law of Thermodynamics - Energy is neither created nor destroyed Second Law of Thermodynamics - With each successive energy transfer, less energy is available to perform work …or energy transfer is not 100% efficient …or entropy increases Energy Living organisms require a constant input of energy, since they are ordered Energy must be supplied from an external source to keep biological processes running The sun’s energy powers living systems Energy that powers Earth’s ecological systems comes mainly from the sun Using solar radiation to produce food Autotrophs (producers) are organisms that use the sun’s energy to produce their own food Plants, algae, cyanobacteria Photosynthesis is the process of turning the sun’s light energy into high-quality chemical energy Sunlight converts carbon dioxide and water into sugars Moving to lower entropy Photosynthesis produces food Chloroplasts are organelles where photosynthesis occurs Contain chlorophyll, a light-absorbing pigment In other words, water and carbon dioxide in the presence of sunlight yields glucose (sugar) and oxygen Glucose serves as primary fuel for all metabolic processes in plant cells Cellular respiration releases energy It occurs in all living things (plants, animals, etc.) Respiration occurs in the mitochondria Organisms use chemical energy created by photosynthesis Heterotrophs are organisms that gain energy by feeding on others Animals, fungi, microbes Cellular respiration splits carbon and hydrogen atoms from the sugar molecule and recombine them with oxygen to create carbon dioxide and water Species to ecosystems A species is a “type” of organism A population is all members of a species living in a given area at the same time A community consists of all of the populations of organisms living and interacting in a particular area An ecosystem is the biological community and its physical environment Species Populations Community Ecosystem Ecosystems An ecosystem is all organisms and nonliving entities occurring and interacting in a particular area Biological entities are tightly intertwined with the chemical and physical aspects of their environment For example, in the Chesapeake Bay estuary: Organisms are affected by water, sediment, and nutrients from the water and land The chemical composition of the water is affected by organism photosynthesis, respiration, and decomposition Energy is converted to biomass Primary production is the conversion of solar energy to chemical energy in sugars by autotrophs during photosynthesis Gross primary production (GPP) is total amount of chemical energy produced by autotrophs Most energy is used to power their own metabolism Net primary production (NPP) is energy remaining after respiration Equals gross primary production – cellular respiration It is used to generate biomass (leaves, stems, roots) Available for heterotrophs Primary productivity of ecosystems Net primary productivity Ecosystems provide vital services All life on Earth depends on healthy, functioning ecosystems Ecosystem services are essential services provided by healthy, normally functioning ecosystems When human activities damage ecosystems, we must devote resources to supply these services ourselves Example: if we kill off insect predators, farmers must use synthetic pesticides that harm people and wildlife One of the most important ecosystem services: Nutrients cycle through the environment in intricate ways Ecological processes provide services Nutrients circulate through ecosystems Nutrients move through the environment in complex ways Matter is continually circulated in an ecosystem Nutrient (biogeochemical) cycle is the movement of nutrients through ecosystems Pool (reservoir) is a location where nutrients remain for varying amounts of time (residence time) Source is a reservoir that releases more materials than it accepts Sink is a reservoir that accepts more than it releases Flux is the rate at which materials move between reservoirs Humans affect nutrient cycling Human activities affect nutrient cycling Altering fluxes, residence times, and amounts of nutrients in reservoirs The water cycle affects all other cycles Water is essential for biochemical reactions and is involved in nearly every environmental system and cycle Hydrologic cycle is the flow of liquid, gaseous, and solid water through the environment Less than 1% is available as fresh water Evaporation is the conversion of liquid to gaseous water Transpiration is the release of water vapor by plants Precipitation is when rain or snow returns water to Earth’s surface Runoff is when water flows into streams, lakes, rivers, oceans Transpiration Water is also stored underground Infiltration is when water soaks down through rock and soil to recharge aquifers Aquifers are underground reservoirs of rock and soil that hold groundwater Water table is the uppermost level of groundwater held in an aquifer Water in aquifers may be ancient (thousands of years old) Hydrologic cycle Most water is stored in the oceans Solar energy continually evaporates water stored in the oceans and land, and distributes water vapor around the globe Condenses over land surfaces, supporting all terrestrial systems Responsible for cell metabolism, nutrient flow in ecosystems, and global distribution of heat and energy The hydrologic cycle Human impacts on the hydrologic cycle Humans have affected almost every flux, reservoir, and residence time in the water cycle Damming rivers slows water movement and increases evaporation Removal of vegetation increases runoff and erosion while decreasing infiltration and transpiration Overdrawing surface and groundwater for agriculture, industry, and domestic uses lowers water tables Emitting air pollutants that dissolve in water changes the nature of precipitation and decreases cleansing effect Carbon cycle Carbon is a structural component of organic molecules and provides metabolic energy Begins with intake of CO2 during photosynthesis. Carbon atoms are incorporated into glucose and then: Remain in plant material until death, eaten, respired, excreted Decomposition returns carbon to the sediment, the largest reservoir of carbon Fossil fuels are carbon sink Oceans are second largest reservoir of carbon The carbon cycle Humans affect the carbon cycle Burning fossil fuels moves carbon from the ground to the air Since mid-1700s, people have added over 275 billion tons of carbon dioxide to the atmosphere Cutting forests and burning fields moves carbon from organisms to the air Less carbon dioxide is removed by photosynthesis Today’s atmospheric carbon dioxide reservoir is the largest in the past 800,000 years The driving force behind climate change The nitrogen cycle involves bacteria Nitrogen is contained in proteins, DNA, and RNA Nitrogen makes up 78% of the atmosphere Nitrogen is inert gas and cannot be used by organisms It needs lightning, bacteria, or human intervention to become biologically active and available to organisms Nitrogen must become biologically available Nitrogen-fixing soil bacteria or lightning “fixes” nitrogen gas into ammonium Nitrogen-fixing bacteria live in legumes (e.g., soybeans) Bacteria then convert ammonium ions first into nitrite ions then into nitrate ions through process of nitrification Plants can take up these ions Nitrite and nitrate also come from pollution Animals obtain nitrogen by eating plants or other animals Denitrifying bacteria convert nitrates in soil or water to gaseous nitrogen, releasing it back into the atmosphere The nitrogen cycle Humans greatly affect the nitrogen cycle Historically, nitrogen fixation was a bottleneck; it limited the flux of nitrogen from air into water-soluble forms Industrial fixation fixes nitrogen on a massive scale Overwhelming nature’s denitrification abilities Excess nitrogen leads to hypoxia in coastal areas Burning forests and fossil fuels leads to acid precipitation, adds greenhouse gases, and creates photochemical smog The phosphorus cycle Phosphorus is a key component of cell membranes, DNA, RNA and ATP No significant atmospheric component Most phosphorus is in rocks Weathering releases phosphorus into water Allowing it to be taken up by plants With naturally low environmental concentrations, phosphorus is a limiting factor for plant growth The phosphorus cycle Humans affect the phosphorus cycle Fertilizer from lawns and farmlands Increases phosphorus in soil Its runoff into water increases phytoplankton blooms and hypoxia Wastewater containing detergents releases phosphorus to waterways