Sweetness Lab
advertisement

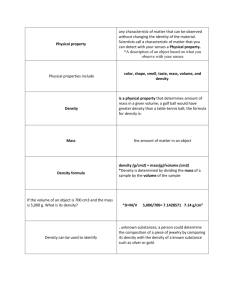
SWEETNESS LAB- SO WHAT MAKES SOMETHING SWEET, ANYWAY???? Watch video clip1 at http://www.pbs.org/thebotanyofdesire/lesson-plan-sweetness.php ***You've been hired by Johnny Appleseed Orchards and the Philadelphia School District to develop a rating scale that measures the sweetness of its apples, relative to soft drinks, artificial and natural sweeteners and sugar solutions of known concentrations. This will help you create an ad campaign to market the orchard's new variety of apples and also modify the overconsumption of sugary drinks by school students. Background Information on sugar substitutes and the physiology of taste: Truvia is a stevia-based sugar substitute developed jointly by Coca-Cola and Cargill. Because it comes from the stevia (http://en.wikipedia.org/wiki/Stevia) plant, Cargill classifies it as a natural sweetener in addition to being a nonnutritive sweetener.[2] It is made of rebiana, erythritol, and natural flavors, which when used as a non-nutritive sweetener is 200 times sweeter than sugar. Rebiana is derived from Stevia leaves by steeping them in water). Since its launch in 2008, Truvia natural sweetener has captured more than 9.5% of the $1.3 billion U.S. sugar substitute market, surpassing Merisant's Equal as the number 3 sugar substitute. Erythritol ((2R,3S)-butane-1,2,3,4-tetraol) is a sugar alcohol (or polyol). In the body, erythritol is absorbed into the bloodstream in the small intestine, and then for the most part excreted unchanged in the urine. Because erythritol is normally absorbed before it enters the large intestine, it does not normally cause laxative effects as are often experienced after consumption of other sugar alcohols (such as xylitol and maltitol) and most people will consume erythritol with no side effects. It is 60–70% as sweet as table sugar yet it is almost non-caloric, does not affect blood sugar, does not cause tooth decay, and is absorbed by the body, therefore unlikely to cause gastric side effects unlike other sugar alcohols. Sucralose is approximately 600 times as sweet as sucrose (table sugar), twice as sweet as saccharin, and 3.3 times as sweet as aspartame. It is stable under heat and over a broad range of pH conditions. Therefore, it can be used in baking or in products that require a longer shelf life. The commercial success of sucralose-based products stems from its favorable comparison to other low-calorie sweeteners in terms of taste, stability, and safety. Common brand names of sucralose-based sweeteners are Splenda, Aspartame is an artificial, non-saccharide sweetener used as a sugar substitute in some foods and beverages. Aspartame is a methyl ester of the aspartic acid/phenylalanine dipeptide. It was first sold under the brand name NutraSweet; since 2009 it also has been sold under the brand name AminoSweet. Saccharin is an artificial sweetener. The basic substance, benzoic sulfilimine, has effectively no food energy and is much sweeter than sucrose, but has a bitter or metallic aftertaste, especially at high concentrations. Saccharin is unstable when heated but it does not react chemically with other food ingredients. As such, it stores well. Blends of saccharin with other sweeteners are often used to compensate for each sweetener's weaknesses and faults - each sweetener masks the other's off-taste. Saccharin is believed to be an important discovery, especially for diabetics, as it goes directly through the human digestive system without being digested. Although saccharin has no food energy, it can trigger the release of insulin in humans and rats, apparently as a result of its taste, as can other sweeteners like aspartame. http://www.edinformatics.com/math_science/science_of_cooking/taste_molecules.htm; www.wikipedia.org For more info: http://www.caloriecontrol.org/sweeteners-and-lite/sugar-substitutes THE MOLECULAR BASIS OF TASTE Taste reception occurs at the apical tip of taste cells that form taste buds. Each onion shaped taste bud has is composed of 50–100 taste cells that possess microvilli. Each single taste bud contains 50–100 taste cells representing all 5 taste sensations. Embedded in the cell membranes of these taste cells are receptor proteins. Each of the taste receptors are transmembrane proteins which function either by physically binding to a flavor ingredient (sweet, bitter and umami) or by acting as a channel to allow ions to flow directly into a taste cell (salty and sour). This interaction triggers a signaling cascade that culminates with signals to the brain through a network of taste nerve fibers. In the case of sweet, umami and bitter tasting it is the size and shape and the chemical groups on a tastant molecule which determine any taste it will have as they will determine its ability to dock onto one or more of the different receptor proteins . A single taste cell seems to be restricted to expressing only a single type of receptor. Schematic representation of taste receptors. http://www.edinformatics.com/math_science/science_of_cooking **most off tastes of high-intensity sweeteners are due to stimulation of the T2R (bitter) family of receptors. Intermolecular forces: “5 types of molecular interactions that can contribute to receptor protein-ligand/sweet tasting molecule interactions. Hydrogen bonding, Van der Waals interactions, salt bridges/ionic bonds, pistacking interactions and hydrophobic interactions. It's easy to speculate H-bonding based on the structures of artificial sweeteners and carbohydrates. Many of the artificial sweeteners and protein sweeteners have aromatic rings, so it's likely that some form of pi-stacking could be used. I know charge is important for binding sweet proteins, so a salt bridge is a possibility, but I'm not sure if modeling has ever predicted it. Van der Waals interactions seem to be everywhere, they are pretty much any interaction not listed above, dipoledipole, for example, seems likely.” Dr. Sarah Lipchock, Monell Chemical Senses Center Pre-Lab Questions: (use the introduction, background information and your notes) 1. How are you able to taste something that is sweet as opposed to something that is bitter? Use the following terms in your explanation: papillae, taste bud, taste cell, receptor 2. How can some foods have more than one taste? (ex. some artificial sweeteners taste both sweet and bitter) 3. Briefly describe each of the following types of intermolecular interactions. Use the websites listed below: http://www.elmhurst.edu/~chm/vchembook/160Aintermolec.html http://en.wikipedia.org/wiki/Intermolecular_force http://www.chemguide.co.uk/atoms/bonding/vdw.html http://itl.chem.ufl.edu/2045/lectures/lec_g.html http://en.wikipedia.org/wiki/Salt_bridge_%28protein%29 http://en.wikipedia.org/wiki/Pi_stacking a. Van der Waal's forces b. hydrogen bonds c. hydrophobic interactions - Materials (per group 4): 50 75 ml Dixie cups 1 Sweet n’ low packet (Saccharin) in 1 Dixie cup water 1 Equal packets (Aspartame, dextrose, maltodextrin) or 1 NutraSweet (aspartame) in 1 Dixie cup 1 Splenda packet (Sucralose) in 1 Dixie cup water 1 Truvia packets (Erythritol, Rebaudioside A) in 1 Dixie cup water Note: manufacturer recommendation states:1packet artificial sweetener=2teaspoons sugar(4g/tsp) 3 – Sugar packets (Sucrose) 12% sucrose solution 6% sucrose solution [mix 5g sucrose/1 sugar packet (check weight - may vary from 2.5-5g) with 1 Dixie cup H2O] 3% sucrose solution Coke in a Dixie cup water in a Dixie cup potato 3 varieties of apples Your Procedure: 1. You need a rating scale of 0-100 for your data to be considered "quantitative". You should assign plain water a rating of 0. Assign the 12% sugar solution a rating of 100. Something else that you then test could be rated > 100. 2. Have everyone in your group taste each substance and rate its sweetness. Taste the 12% sugar solution first. Also record any observations about its sweet characteristics or other tastes that you experience. **NOTE: You may want to re-taste the 12% and 0% before tasting the food group and then before the artificial sweetener group. Describe how you were able to assign sweetness values to the apples and other foods: Results: Substance Sweetness rating water 0 12% sucrose 100 6% sucrose 3 % sucrose Apple 1 ( ) Apple 2 ( ) Apple 3 ( ) potato Coke Candy (__________________) Sweet n Low Equal / NutraSweet Splenda Truvia Other comments? Group Data: Enter your group's ratings on the Excel data spreadsheet . 1. Copy the spreadsheet data table and paste it below: Data Table: 2. Create a graph that best illustrates your personal data and use the preformatted graph for your group's data, using MS Excel. Copy and paste them below: Discussion Questions: 1. Look at the standard deviation for each item that was rated. How reliable is the data for each group of items (ex. % sugar solutions, apples, artificial sweeteners)? 2. Is your data consistent with the sweetness ratings described in the Introduction? Explain. 3. Why might one person taste something as sweeter than another person? Think about the structure of those molecules, the structure of the sweet receptors, the types of interactions between sweet tasting molecules and the sweet receptor, and the "anatomy" of taste. (*sweet receptors are proteins that your cells build, based on the instructions in your DNA - your genes for sweet receptors. We all have genes that make the enzymes that help build the pigments that give us hair color for example, but my genes might have slightly different information than your genes. My genes also determine how many hair follicles I have and how "hairy" I am. Apply this information to sweet receptors to help answer the question.) 4. Sugars are not always good news, especially for someone who has problems handling them in their body. What are the causes (not risk factors) and effects of diabetes? http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002194/; http://www.diabetes.org/ 5. What is the specific role of insulin in the body? http://en.wikipedia.org/wiki/Insulin 6. What is 1 advantage and 1 myth of artificial sweeteners? http://www.caloriecontrol.org/health-professional-library/benefits-of-low-calorie-sweeteners http://www.caloriecontrol.org/sweeteners-and-lite/sugar-substitutes http://www.caloriecontrol.org/articles-and-video/myth-of-the-month-0 7. How might your rating scale be useful in schools or society in general? 8. Come up with a 1-2 sentence slogan / ad campaign to promote what you've been hired to do! some great stuff on the biochemistry of tastes: http://www.cbsnews.com/8301-18560_16257330816/the-flavorists-tweaking-tastes-and-creating-cravings/?tag=contentMain;cbsCarousel Teaching Notes: http://www.ehow.com/facts_7190808_much-sugar-apple-have_.html Size Apples average about 3 grams of sugar per ounce. According to the California Apple Commission, a medium apple weighing 5.5 oz. has 16 grams of sugar. The Washington Apple Commission states that a large, 8-ounce apple contains 25 grams of sugar. Variety Some apple varieties are sweeter than others. Red and Golden Delicious have 11 to 15 percent sugar. Granny Smith apples have 12 to 18 percent sugar. The sugar content of Gala apples is 14 to 16 percent. Fuji is one of the sweetest varieties with 16 to 18 percent sugar. Carbohydrates Sugar accounts for about 73 percent of the carbohydrates in an apple. Fiber makes up most of the remaining carbohydrates. A medium apple contains about 5 grams of fiber.