Unit C Topic 2
advertisement

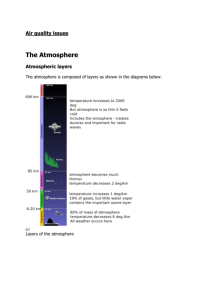
Science 9|2013 Name:_______________ Date:_______________ Unit C: Environmental Chemistry Topic 2 2.0 The quality of chemicals in the environment can be monitored. What is the ozone layer and why is it so important? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ What is environmental monitoring? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 2.1 Monitoring Water Quality During the summer, ____________________ can cause lake water to become ____________. Large quantities of algae reduce the __________________ in the lake, which can affect the different organisms that live in the lake. For example, ____________ are one of the first fish to _________ when ___________ concentration decreases. __________ is not a good indicator of water quality. For example, lakes affected by ______________________ are crystal clear and lifeless. What are the five categories of water use? 1.___________________________ 2.___________________________ 3.___________________________ 4.___________________________ 5.___________________________ Biological Indicators What is a biological indicator? Give two examples. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Science 9|2013 What are invertebrates? How are aquatic invertebrates used to indicate water quality? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Aquatic Environments Why does diversity decrease as acidity increases and dissolved oxygen decreases? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Chemical Factors that Affect Organisms __________________ is made up of only ___________ molecules, but water in the _____________ is never completely pure. It can contain many different ___________________ and _____________________. The concentration of these compounds affects ____________________________. List the six indicators of water quality. 1._________________________ 2._________________________ 3._________________________ 4._________________________ 5._________________________ 6._________________________ Measuring Chemicals in the Environment What is parts per million? ________________________________________________________________________ ________________________________________________________________________ Write the statement for parts per million as a ratio, fraction, and equation. Science 9|2013 Dissolved Oxygen What factors influence the level of dissolved oxygen in water? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ What range of dissolved oxygen are needed by the following aquatic invertebrates: a). most invertebrates ___________ b). midge larva _______________ c). stoneflies _______________ d). freshwater shrimp _________________ Phosphorus and Nitrogen Content High phosphorus and nitrogen in water can affect dissolved oxygen levels. How do large amounts of phosphorus and nitrogen enter into a water system? _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ What happens when large amounts of phosphorus and nitrogen enter into a water system? _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ Draw the three pictures of the effects of increased amounts of phosphorus and nitrogen. Science 9|2013 Acidity What is spring acid shock? Why can it be a major issue? __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ Pesticides Describe the issue of pesticides and resistance in the pests they target? __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ Science 9|2013 What is toxicity? __________________________________________________________________ __________________________________________________________________ Measuring Toxicity What is a toxin? __________________________________________________________________ __________________________________________________________________ What is LD50 and what does it mean? What outcome are scientists testing for when they are measuring LD50? __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ Heavy Metals In the 1950s, many people in ____________________ were becoming sick and dying from a mysterious _____________ that affected their __________________. It was discovered that the _________________ disease was linked to eating local _______ that contained high quantities of ________________. The _______________ was traced back to a ____________________________ that had been dumping its ______________ into the ocean. People who caught and ate the fish from this area were affected by ______________________________. The symptoms of __________________ include numbness of arms and legs, _______________________, nerve damage, and brain damage. What are heavy metals? Provide three examples. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ List examples of situations that increase the availability of heavy metals. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Science 9|2013 2.2 Monitoring Air Quality What percentages of the air are: a). nitrogen __________ b). oxygen _________ c). argon ________ d). carbon dioxide __________ What are some signs of poor air quality? ________________________________________________________________________ ________________________________________________________________________ Sulfur Dioxide Where does sulfur dioxide come from? ________________________________________________________________________ ________________________________________________________________________ How do industrial and electrical generating plants reduce sulfur dioxide? ________________________________________________________________________ ________________________________________________________________________ Nitrogen Oxides Nitrogen oxides are written as NOx(g). Why is it written with an ‘X’? ________________________________________________________________________ Carbon Monoxide How does carbon monoxide form? ________________________________________________________________________ ________________________________________________________________________ Why is carbon monoxide so dangerous? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Ground-Level Ozone How is ozone both beneficial and harmful to organisms? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Science 9|2013 What is ozone? ________________________________________________________________________ ________________________________________________________________________ What are VOCs and what are the dangers of VOCs? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 2.3 Monitoring the Atmosphere Two international issues are related to carbon dioxide concentration. What are they? ________________________________________________________________________ Carbon Dioxide as a Greenhouse Gas Why is carbon dioxide not considered a pollutant? ________________________________________________________________________ ________________________________________________________________________ Describe the greenhouse effect. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Describe the enhanced greenhouse effect, and how it contributes to global warming. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Draw a picture of the greenhouse effect and the enhanced greenhouse effect. Science 9|2013 What are some ways that countries have started reducing their carbon dioxide emissions? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ The Ozone Layer How does the ozone layer protect the Earth from ultraviolet radiation? _____________________________________________________________________ _____________________________________________________________________ What are some consequences of holes in the ozone layer? _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ The Role of Chlorofluorocarbons Scientists have concluded that the ______________ of the ___________________ is caused by chemicals called _________________________, which are used in refrigerators, aerosol cans, and fire extinguishers. ________________________ slowly move from lower atmosphere to upper atmosphere. _____________________ breaks them down into substance, such as _______________________. ______________________ atoms react with the _____________________________ to form oxygen molecules. One ____________________ can remove 100 000 ___________________. The ozone hole over the __________________________ is due to the extremely ___________________________, which cause _______________________ to form in the upper atmosphere. These _______________________________speed up the reaction that removes ___________________________________. Many countries have recognized that CFCs are a danger to the _______________, and they have signed international ________________________ to reduce the use of CFCs.