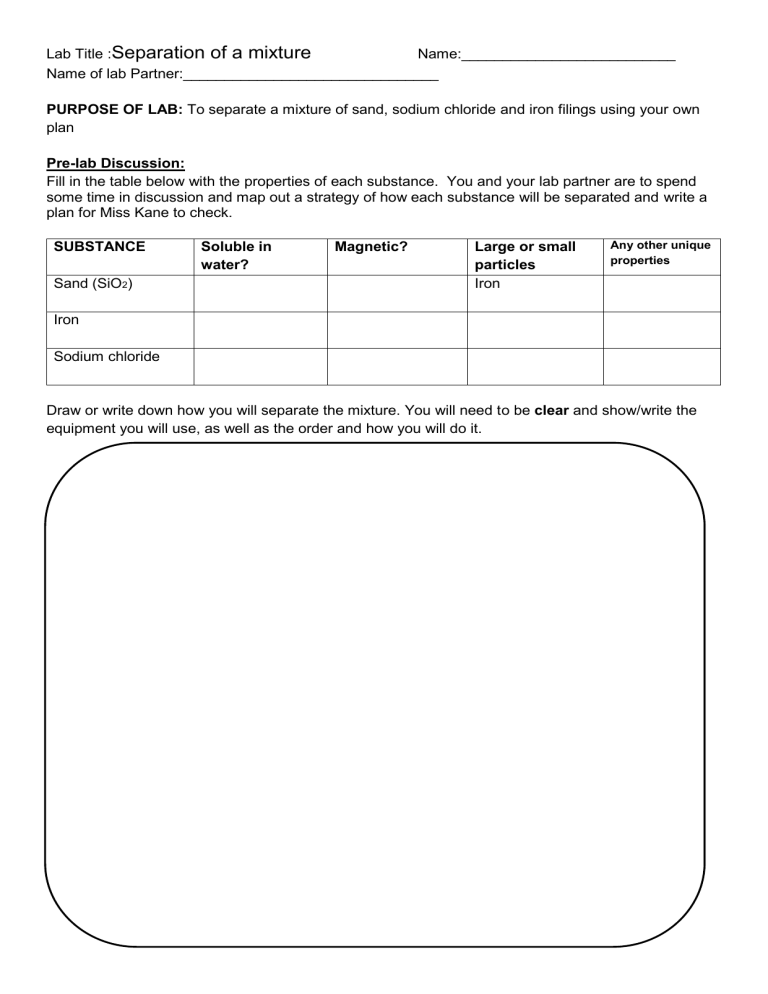
Lab Title :Separation of a mixture Name:__________________________ Name of lab Partner:_______________________________ PURPOSE OF LAB: To separate a mixture of sand, sodium chloride and iron filings using your own plan Pre-lab Discussion: Fill in the table below with the properties of each substance. You and your lab partner are to spend some time in discussion and map out a strategy of how each substance will be separated and write a plan for Miss Kane to check. SUBSTANCE Sand (SiO2) Soluble in water? Magnetic? Large or small particles Iron Any other unique properties Iron Sodium chloride Draw or write down how you will separate the mixture. You will need to be clear and show/write the equipment you will use, as well as the order and how you will do it. Results (Write down what happened) Discussion Relate the properties of the substances (in your table above) to why you could use that method of separation. Eg Marbles are very large, so we could sift them and they would not go through the sieve whereas the other materials are small and can fit through the gaps. This would separate the marble from the rest of the mixture. Questions 1. What 2 other elements are magnetic? 2. What do the following words mean? a) Soluble b) Solvent c) Insoluble 3. Which chemical was soluble in water? 4. Which chemical was insoluble in water? 5. Use the solubility rules from the table to work out whether the following are soluble in water or not. Sodium chloride ______________________ Silver chloride ______________________ Sodium carbonate ______________________ Sodium sulphate ______________________ Magnesium chloride ______________________ Copper hydroxide ______________________ MARK SCHEDULE Not achieved- does not separate all 3 substances Achieved- separates all 3 substances Merit – able to link substance properties to being able to separate Excellence able to use the words soluble, insoluble, magnetic and evaporation correctly and links to substances properties




