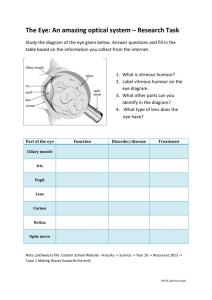
0_Krakova_Yelena - INDIGO @ UIC
advertisement

Development of a Multi-Electrode Electroretinography System and Characterization of meERG Signals in Rat BY YELENA KRAKOVA B.S., University of Illinois at Chicago, Chicago, 2005 THESIS Submitted as partial fulfillment of the requirements for the degree of Master of Science in Bioengineering in the Graduate College of the University of Illinois at Chicago, 2012 Defense Committee: John Hetling, Advisor James Patton Simon Alford, Neuroscience ii TABLE OF CONTENTS Chapter Page 1. Brief overview………………………………………………………………………………1 1.1. Problem statement …………………………………………………………………..... 1 1.2. Specific Aims……………………………………………………………………….....1 1.3. Summary of results………………………………………………………………........ 2 2. Introduction ………………………………………………………………….....................3 3. Background ……………………………………………………………………………… 4 3.1. Overview of anatomy / physiology of the eye………………………………………. 4 3.2. The ERG and its origins……………………………………………………………... 6 3.3. A- and B-wave……………………………………………………………………….. 6 3.4. ISCEV standard…………………………………………………………………….... 8 3.5. Current electrode types……………………………………………………................. 9 4. Existing methods for mapping retinal function …………………………………………..10 4.1. Perimetry – HVF, Goldman………………………………………………………….11 4.2. mfERG ……………………………………………………………………………….11 4.3. Potential advantages of meERG-based approach to mapping retinal function……... 12 4.4. Commercially available ERG stimulus sources……………………………………...12 Methods and Results by Specific Aim 5. Specific Aim 1………………………………………………………………………….....14 5.1. Specific Aim 1 methods……………………………………………………………...15 A. Initial design of the stimulus source……………………………………………....15 B. Star stop …………………………………………………………………………..17 5.2. Specific Aim 1 Results……………………………………………………………….18 A. Light source calibration………………………………………………………...…18 B. Luminance profiles through time………………………………………………….19 ii iii TABLE OF CONTENTS (continued) 6. Specific Aim 2…………………………………………………………………………….22 6.1. Specific Aim 2 methods……………………………………………………………22 A. Lens assembly procedure………………………………………………………....24 B. Revision of lens design…………………………………………………………....25 i. Analysis of rat eye size vs. age…………………………………………....26 ii. Measurement Procedure using DinoCapture 2.0 version 1.3.2…………. .26 iii. Measurement Procedure using ImageJ……………………………………28 iv. Depth of lens cup………………………………………………………….29 v. Width of lens voids………………………………………………………..30 C. Lens Filling Procedures………………………………………………………….. 31 i. Saline………………………………………………………………………31 ii. Saline lens filling procedure………………………………………………32 iii. Saline lens cleaning and storage procedure……………………………….33 iv. Poly-Ethylene Glycol (PEG)………………………………….....................33 v. PEG fabrication and lens filling procedure……………………………….34 vi. PEG lens storage procedure………………………………………………35 6.2 A. B. C. D. Specific Aim 2 results……………………………………………………………..35 Analysis of rat eye size vs age…………………………………………………….35 Depth of lens cup………………………………………………………………….37 Width of lens voids………………………………………………………………..38 Lens filling procedures……………………………………………………………38 7. Specific Aim 3 ……………………………………………………………………………40 7.1. Specific Aim 3 methods……………………………………………………………40 A. Recording protocol………………………………………………………………..41 B. Analysis protocol………………………………………………………………….43 7.2. Specific Aim 3 results……………………………………………………………...44 A. Typical meERG response…………………………………………………………44 B. Inter-animal variability……………………………………………………………49 i. A-wave by ring…………………………………………………………….49 ii. B-wave by ring…………………………………………………………….52 C. Spatial Differences……………………………………………………………...…54 D. Test-retest variability (same animal)…………………………………………........56 8. Discussion…………………………………………………………………………………60 iii iv TABLE OF CONTENTS (continued) 8.1. Summary of Results by Specific Aim………………………………………………..61 A. Summary of Results: Specific Aim-1……………………………………………..61 B. Summary of Results: Specific Aim-2……………………………………………..62 C. Summary of Results: Specific Aim-3……………………………………………..64 9. Cited Literature……………………………………………………………………………66 10. Appendix………………………………………………………………………………….68 10.1. Standard operating procedures………………………………………………………68 A. Lens Assembly………………………………………………………………………..68 B. Measurement Procedure using DinoCapture 2.0 version 1.3.2 ………………………68 C. Measurement Procedure using ImageJ ……………………………………….............69 D. Saline lens filling procedure…………………………………………………………..69 E. Saline lens cleaning and storage procedure…………………………………………..70 F. PEG lens filling procedure……………………………………………………............70 G. PEG fabrication procedure……………………………………………………............71 H. PEG lens storage procedure…………………………………………………………..71 I. Recording Protocol …………………………………………………………………...72 J. Analysis Protocol……………………………………………………………………..72 iv v LIST OF FIGURES Figure Page 1. Anatomy of the human eye……………………………………………………………5 2. A typical flash-evoked corneal ERG …………………………………………………7 3. Schematic current source model of the eye…………………………………………...7 4. Photograph of Burian-Allen and DTL electrodes…………………………………….10 5. Schematic of the spatially –homogenous light –flash stimulus source……………….16 6. Star stop ………………………………………………………………………………16 7. Light diffuser dome…………………………………………………………………..16 8. Star stop with ring pattern overlay……………………………………………...........17 9. Star stop flow chart……………………………………………………………………18 10. Luminance profiles through time……………………………………………………..21 11. Photo and schematic of rat lens……………………………………………………….23 12. Photo of the lens assembly table……………………………………………………...25 13. Screen capture of the measurement procedure using DinoCapture…………………..28 14. Schematic of base lens and a photo of the base lens…………………………………30 15. Rat eye radius measurements plotted from the age of 24 to 66 days…………………36 16. Photos of the modified and original depth of lens……………………………………37 17. Impedance of channel 77 on the CLEAr Lenstm..........................................................40 18. A photograph of an anesthetized rat during a recording……………………………..43 19. Position plot and electrode map………………………………………………………45 20. Normalized standard deviation of the amplitudes from Table 3 …………………….47 21. A wave amplitudes from 22 experiments averaged together…..................................48 v vi LIST OF FIGURES (CONTINUED) 22. The mean divided by the standard deviation of Figure 21 plotted……………………48 23. Normalized a- wave amplitude ratios from 8 animals at 6 and 7 weeks of age………51 24. Normalized b-wave amplitude ratios from 8 animals at 6 and 7 weeks of age ………53 25. Probability distribution of a-wave amplitude ratios for 18 experiments …………….55 26. Probability distribution of a-wave amplitude ratios for 18 experiments averaged together……………………………………………………………………………….56 27. A wave amplitude ratios from 8 animals at 6 and 7 weeks of age …………………..58 28. Averaged a-wave amplitude ratios from 8 animals at 6 and 7 weeks of age…………60 vi vii LIST OF TABLES Table Page 1. Light source calibration data…………………………………………………………..20 2. Light source calibration table with light from filters…………………………………..41 3. Mean a- and b-wave amplitudes with all electrodes averaged………………………...46 vii viii LIST OF EQUATIONS Equation Page 1. Normalized ring averages…………………………………………………. 2. Spatial ring ratios…………………………………………………………… viii ix LIST OF ABBREVIATIONS ERG Electroretinogram mfERG Multi-focal electroretinogram meERG Multi-electrode electroretinogram SNR Signal to noise ratio PMMA Polymethyl-methacrylate PEG Poly-ethylene-glycol Sc cd s m-2 Scotopic candela seconds per meter squared CLEAr Lens™ Contact lens electrode array ISCEV International Society for Clinical Electrophysiology of Vision NEVL Neural engineering vision laboratory HVF Humphrey visual field ix 1. Brief overview Multi-electrode electroretinography (meERG) is a new technique being developed in the Neural Engineering Vision Laboratory at UIC. The meERG is a simultaneous measure of potentials at several locations on the corneal surface in response to a photic stimulus. It is anticipated that analysis of these potentials will reveal spatial information about the health of the retina, and thus may prove useful in early diagnosis of eye diseases, such as glaucoma and diabetic retinopathy. 1.1 Problem statement While the meERG technique is being developed for clinical use, there is a great deal that can be learned in animal studies. In particular, the NEVL lab is working to characterize the sensitivity and specificity of the technique for detecting laser-induced retinal lesions in rats. The main goal of this thesis was to support this effort by improving the design of the stimulus source and the contact lens electrode array, and by doing the initial characterization of the meERG signals recorded from normally-sighted rats. With this in mind, the following aims were addressed. 1.2 Specific Aims I. To optimize a full-field, spatially-homogenous light-flash stimulus source suitable for use with rats. -1- -2- II. To improve the design and function of the rat-sized meERG Contact Lens Electrode Array (CLEAr Lens™), with the goals of increasing the yield of functional electrodes on each lens and improving the quality of the recorded signal. III. To record the meERG signal from normal rats and to characterize this relatively novel signal with regard to test-retest and inter animal variability and to quantify spatial differences in meERG potentials across the rat cornea Note: CLEAr Lens™ is a trademark of RetMap, Inc., a start-up company founded by NEVL alumni to facilitate development and distribution of meERG technology. 1.3 Summary of results: A full-field light flash stimulus source for use in rats was optimized and characterized, and found suitable for the planned experiments (it is currently in routine use). The lens design, as well as the procedure for preparing the lens for recording, were both significantly improved; the lens preparation procedures developed here have been adopted for the human-version of the CLEAr Lens™ as well. The meERG responses were analyzed for spatial differences in amplitudes; the typical standard deviation of amplitudes across the corneal surface was 2-3% of the mean, indicating fairly uniform potnetials for normally-sighted rats. Inter-animal variability between aand b-wave amplitudes was found to be high, but consistent with conventional ERG results. Regarding test-retest variability, a measure related to the spatial distribution of a-wave amplitudes was found to be significantly different in three of eight animals when comparing measurements made one week apart; this seems to indicate a degree of procedural variability inherent in this relatively new measurement. 2. Introduction There is a high prevalence of degenerative retinal diseases that affect the peripheral retina, such as glaucoma and retinitis pigmentosa, that currently have limited means of early detection. Methods that are available today include the Humphrey Visual Field test and the multi-focal electroretinogram (ERG). These methods are only capable of detecting the disease once it progresses into the central visual field and either have a long duration time or require the patient to have good visual acuity. The meERG has the potential to overcome these challenges by probing the entire retina in a short duration time and being independent of good visual acuity. The purpose of the work presented here is to support the development of the new multi-electrode electroretinogram (meERG) technique by improving the design of the full-field spatiallyhomogeneous stimulus source and characterizing the meERG signals collected from rats to begin the formation of a normative data set. The meERG is a means of detecting potentials on the cornea at several locations simultaneously. The characterization of the meERG helps set the foundation for the spatial differences present in corneal a and b wave amplitudes in normal rats. Simultaneously recorded amplitudes at multiple locations on the cornea have been analyzed on a limited basis in the literature; spatial variance will be the focus in this thesis. -3- 3. Background 3.1 Overview of anatomy/physiology of the eye The retina is a layer of several cell types that lines the inner surface of the eye. The human retina is approximately 0.5 mm thick (webvision, 2012), and contains approximately 55 different cell types, each with a different function (Masland, 2001). A cross section of the retina shows photoreceptors, ganglion cells, muller cells, amacrine cells, horizontal cells and bipolar cells. Figure 1 shows these major cell types. There are three layers of nerve cell bodies and two layers where synapses occur. The layer that is furthest from the cornea contains the rods and cones which make up the two types of photoreceptors. In most mammalian species, rods outnumber cones by approximately 20-fold (Masland, 2001). Starting at the outer most part of the retina, the rods and cones are followed by the outer nuclear layer which contains bipolar and horizontal cells. Amacrine cells make up the inner nuclear layer which is followed by the ganglion cells and their axons. The nerve fiber layer forms the vitreous surface of the retina and is the first retinal layer to receive light entering the eye. -4- -5- A) B) Aqueous humor Vitreous humor Figure 1: (A) Cross section of a human eye (B) Schematic cross section of a mammalian retina. The outer most part of the retina contains the rods and cones followed by the outer nuclear layer, outer plexiform layer, inner nuclear layer and the inner plexiform layer (webvision, 2012). When light enters the eye, it must pass through the cornea, aqueous humor, lens, vitreous humor and several cell layers present in the retina before reaching the photoreceptors. The central part of the retina has a high density of cones while the peripheral part of the retina is rod dominated (Purves, 2001). The cones are responsible for color vision at higher light levels while the rods are capable of functioning in minimal light and hence are primarily responsible for night vision (webvision 2012). When a photon enters the eye and reaches the retina the molecule rhodopsin, a photo pigment in the rod photoreceptors, undergoes a conformation change that through a signal cascade results in the hyperpolarization of the cell. The hyperpolarization reduces the release of the neurotransmitter glutamate at the synaptic cleft. Glutamate causes the depolarization of the ON-bipolar cells and the hyperpolarization of the OFF bipolar cells. OFF and ON bipolar cells occur in approximately equal numbers (Masland, 2001). The signal then travels to the ganglion cells where an action potential occurs to be further propagated through the optic nerve into the primary visual cortex in the brain for additional processing. 3.2 The ERG and its origins The electroretinogram is a bioelectric signal originating at the retina that can be recorded at the surface of the cornea. It is a test used in basic research to assess the state of the retina in diseased as well as healthy eyes. It is used in human patients as well as laboratory animals. Typically, an electrode is placed on the cornea and a light flash is produced which elicits a recordable waveform. A full-field, spatially homogenous stimulus source such as the Ganzfeld dome and electrodes types such as the Burian-Allen, JET and DTL are commonly used for recording the ERG (Figure 4). The ERG is recorded following the ISCEV standard (Hood et al., 2012). This standard states that the recording electrode impedance must be measured and the patient dark adapted based on the type of recording that is to be performed. The appropriate stimulation levels for recording rod or combined rod-cone responses are given with appropriate interval times between stimuli. The standard includes guidelines for averaging as well as reporting the recorded information. 3.3 A- and B wave Several cell types contribute the recordable ERG waveform; for this introduction a-wave and bwave origins are described below. Figure 2 is an example of a typical flash evoked corneal ERG showing the a- and b-wave. The a-wave represents the light response of the photoreceptors and reflects the change in the circulating currents due to light absorption. The initial negative slope of the ERG is the a-wave and would have a much longer duration if the photoreceptors were to be isolated from the rest of the retina. Due to the intrusion of the b-wave, only a small portion of the a-wave is typically visible (Lamb & Pugh, 1992). With the decrease in glutamate post light stimulus, the ON-bipolar cells activate and depolarize, responsible for the positive peak seen in the ERG waveform. 1200 b-wave 1000 800 µV 600 400 200 0 -200 0 -400 -600 50 100 150 200 250 300 350 a-wave ms Figure 2: A typical flash-evoked corneal ERG recorded with the CLEAr Lens™ in rat. Waveform recorded on electrode channel 83 at flash strength of 116 sc cd s m-2 delivered at 50.4 ms. (experiment 20111123-A) Figure 3: Schematic current source model of the eye, (webvision, 2012). 400 A mammalian eye can be represented with the circuit diagram in Figure 3. A light stimulus produces an electrical current that can be divided into local and remote pathways as illustrated by pathway A and B. Hence two electrodes are used to record the ERG, one placed on the cornea and the other which serves as the reference is commonly placed on the cheek. The ERG is a potential change that is related to the light induced electrical activity within the retina (webvision, 2012). As the retina deteriorates due to a pathology, the light-induced response of the retina is reduced and so are the waveforms of the ERG. 3.4 ISCEV standard (2011) The International Society for Clinical Electrophysiology of Vision (ISCEV) promotes clinical electrophysiology of vision and defines recording standards to promote cooperation and communication among the field. This standard was followed as closely as possible for the rat recordings. The pupils are to be dilated maximally and the pupil size noted. The electrode type such as the Burian-Allen or the DTL should be used for recording the ERG. The electrode stability and impedance are to be measured. For recording the rod response the patient is to be dark-adapted for a minimum of 20 minutes. The stimulus is a dim white flash of 0.01 cd s m-2 with a minimum interval of 2 seconds between flashes. For recording the combined rod-cone response a white light of 3.0 cd s m-2 is to be used. There should be at least a 10 second interval between stimuli. Averaging the recorded signals is not required when using the recommended electrode types. Patient identification, date as well as any events that deviate from the ISCEV standard are to be reported. 3.5 Current electrode types The Burian-Allen electrode is a speculum type structure that holds the eye open while a contact lens with a wire on its periphery floats on the retina. The Burian-Allen is bipolar and reusable with reference electrodes built in. This type of electrode is size specific and in order to fit the patient properly, it is available in four sizes. (Figure 4A) The Jet electrode is a contact lens with an embedded gold foil electrode; it is a mono-polar electrode that comes in one size only. This contact lens electrode is disposable. The DTL electrode is a silver-nylon thread wire that lies along the lower lid and sclera or cornea. This type of electrode is not limited by patients eye size and is disposable. It is held in place by an adhesive located on the white foam pads and is ideal where user comfort is an issue. (Figure 4B) A) B) Figure 4: A) Photograph of a Burian-Allen electrode. B) Photograph of a DTL electrode. 4. Existing methods for mapping retinal function The most recent report of an meERG recording in literature was performed by Sundmark (1959) using a 9 electrode “contact glass.” The Neural Engineering Vision Laboratory (NEVL) at UIC is investigating the process of electrophysiological mapping of retinal function with the multielectrode CLEAr Lens™. The meERG is comparable to techniques developed for 3D mapping of the brain and heart from body surface potentials (He & Wu, 1999). Several methods exist that map retinal function in patients but have several limitations. These methods are briefly discussed below. - 10 - 4.1 Perimetry- Humphrey visual field, Goldman perimetry The Humphrey visual field (HVF) is a test which requires the patient to fixate on a small target. Spots of light are introduced at defined locations within the visual field and the patient responds when he or she sees them. The most common variation of the HVF technique is the 24-2 test which tests the central 24 degrees of the visual field with a 20 degree target. The test is restricted to the central visual field since visual acuity in the periphery is limited and the test becomes inaccurate. The Goldman visual field test is a test that measures a patients peripheral vision manually. A test target is projected at a single location and intensity is increased until the patient is able to see it. The target is moved to the central visual field from the periphery and feedback from the patient is recorded. The primary problem of these two tests is that they rely on the patient’s feedback and are thus not objective. 4.2 Multifocal electroretinogram The multifocal electroretinogram (mfERG) is a means of applying a pattern stimulus in order to elicit a response from various areas of the retina simultaneously, developed by Sutter and Tran, (1992). The recording is done with a standard ERG electrode. The data is analyzed and correlated to an appropriate area of the retina via a mathematical technique. This test requires the patient to have good central vision as the test probes 30-40 degrees of the central visual field and can take a long time (about 8 minutes per eye) to perform. This test is often used to assess the retinas of patients with retinitis pigmentosa, glaucoma and macular degeneration. A weakness with the mfERG is that the recordings have uncertain correlation to the biological events that occur at the retina and the computed waveforms are open to interpretation (Hood et al., 2002). 4.3 Potential advantages of meERG-based approach to mapping retinal function The fist account of the measuring ERG potentials at various locations simultaneously was by Sundmark (1959), followed by Cringle (1986). Recordable differences in ERG amplitudes based on location of the contact electrode were first noticed by McAllen et al. (1989), and confirmed by Hennesey and Vaegan (1995) that different electrode types yielded varying recorded amplitudes. The meERG is essentially means of collecting corneal potentials at several locations simultaneously in response to a full field light stimuli. The meERG is a noninvasive test that probes the entire visual field and is likely to be independent of the visual acuity of the patient. The test can be applied with varying stimuli in order to analyze various cell types or functional pathways. The meERG results in waveforms that directly reflect the function of the retina. This test is quick (less than a minute per eye) and yields spatial information about corneal potentials and has the potential to yield spatial information about the retina with the use of models (currently being developed) in solving the inverse problem. 4.4 Commercially available ERG stimulus sources The Ganzfeld stimulus source is a full-field light source which allows for control of background illumination and the ability to vary stimulus intensity. The Ganzfeld is typically a dome which fills the visual field and produces a uniform luminance in order to illuminate the retina homogeneously. Despite several attempts via personal communication with LKC Technologies Inc., the manufacturer of the MGS-1 Ganzfeld stimulator as well as by Diagnosys LLC, the manufacturer of the handheld Ganzfeld (ColorBurst) no quantitative information was disclosed about the spatial variance of their products. This made it difficult to compare our results to the commercial standard. The relationship between the source and retinal illuminance is believed to be approximately direct, that is if a Ganzfeld source is used, the resulting light distribution is nearly homogenous over the whole retina. The homogeneity of the light distribution on the retina is not influenced by the size of the pupil or the shape of the optical features (Kooijman, 1983). Retinal illuminance has previously been measured in human as well as rabbit eyes. Retinal illuminance decreases only slightly toward the periphery when measured in human and rabbit eyes upon illumination with a Ganzfeld (Kooijman, A. & Witmer, F., 1986). Since the relationship is considered to be direct, although quite difficult to measure without error the use of a Ganzfeld source will be necessary to stimulate the retina homogeneously. Methods and Results by Specific Aim 5. Specific Aim 1 - To optimize a full field, spatially –homogenous light –flash stimulus source for use with rats. The distribution of the meERG recorded corneal potentials is affected by the spatial distribution of the source of the signal, the retina. Since the ultimate goal is to understand the activity of the retina and reveal areas of abnormal response to stimulus, it is critical to know the distribution of the stimulus across the retina. Ideally, the stimulus source would produce a uniform, spatiallyhomogenous distribution of light across the entire retina. Since it is difficult to measure the exact amount of light that hits the retina, it is approximated that it is proportional to that of the light source. Assuming that the light source is uniform, it is believed that the retina will be illuminated uniformly as well. For this reason it is critical to use a well-characterized stimulus source. Instead of purchasing a commercial stimulus source, one was fabricated in the lab in hope that we would obtain a level of spatial homogeneity that exceeded the current gold-standard commercial units. Since this data was not accessible from commercial companies, the goal of the fabrication process was to create a source with uniform luminance. - 14 - 5.1 Methods A. Initial design of the stimulus source An initial design for the stimulus source was created by Dr. Hetling. This consisted of a 14 x 14 x 20 in plywood box with a series of shelves inside and a light source on top and can be seen in Figure 5A, B. The challenge to meet Specific Aim 1 was to optimize the diffusers and stops between the flash lamp and the back-lit dome in order to achieve a source, which is the concave surface of dome, that was of uniform luminance (+/- 5%). A flash head (2140-C Novatron) was used to produce a flash with an ̴ 0.88 msec duration. A series of four diffusers encased in a plywood box were installed. The first three diffusers (A, B, C respectively) were made using sandblasted polymethyl-methacrylate (PMMA) that was sandblasted on both sides. These were placed in filter slots A and B. Filter slot B contained two sheets of PMMA, the one on top was sandblasted on both sides while the bottom one was a diffuser sheet cut to size that is typically used in fluorescent light fixtures. Filter slot C had a single sided sand blasted PMMA sheet and slot D had the star stop, see Figure 5A. The light was measured from 0 to 70 degrees in 10 degree increments from the normal using a photometer (International light, model IL-1800, with SED #100 photo diode detector, R#485 radiance barrel and the ZCIE#19555 scotopic filter), Figure 7, which was calibrated to scotopic candela seconds per meter squared (sc cd s m-2). This detector was placed on a custom mount that allowed the targeting of +/- 70 degrees of dome surface. Figure 5(B) is a photograph of the stimulus box with the door open and a light turned on. In order to further optimize the light source to the desired luminance profile, a “star stop,” a photography stop in the shape of a star was developed for filter slot D. Figure 6. The process of adjusting the star stop is represented as a flow chart in Figure 8. Figure 10 demonstrates the luminance profile as it evolved through the fabrication process. A) B) Figure 5: (A) Schematic of the spatially–homogenous light–flash stimulus source for use in rats with the photometer detector in place. (B) Photo of the stimulus source for use in rats. Courtesy of Hadi Tajalli. 12 in Figure 6: Star stop, made out of PMMA and aluminum tape. When the star stop is added to the light stimulus box it produces the final luminance profile as seen in Figure 10. Figure 7: Schematic of the light diffuser dome with the photometer detector showing the method used to measure the luminance at ten degree increments. B. Star Stop The star stop design was based on the following goal, to bring the luminance at the measured degree intervals as close to the lowest measured value as possible. The luminance was measured at ten degree intervals starting from zero degrees from the vertical to 70 degrees and averaged, see Figure 7. The PMMA sheet was subdivided into 6 concentric circles drawn from the center of the PMMA with the radii illustrated in Figure 8. Opaque aluminum tape was then added strategically, first perpendicular to the line dawn in each ring and then parallel. The following flow chart outlines the process. Figure 8: Schematic ring pattern overlaid on star stop. The overlaid ring pattern was used in the fabrication process of the star stop. The ring pattern allowed for the division of the PMMA sheet into zones that were later coupled with the eight degree increments that were measured. Figure 9: Star stop optimization process. The flow chart describes the procedure used to fabricate the star stop to produce uniform luminance as measured by the photometer. 5.2 Results A. Light source calibration A uniform full-field stimulus is necessary to achieve uniform illuminace of the retina so it was our goal to create a stimulus source as uniform as possible. Comparing the measured luminance of the source to a flat line (the ideal luminance profile), a sum of squares was calculated as a measure of ‘flatness’ and uniformity of luminance; the lower the value the more uniform the luminance is. The formula for the sum of squares is listed in Table 1. This data is based on five flashes averaged per degree interval. The sum of squares (the sums of the square differences) divided by the average of the light distribution before the star stop was 16,414. After the star stop was applied, the sum of squares was reduced to 7,129. See Table 1 for reference. The addition of the start stop decreased the variance of the light distribution from 66% to 22%. The table shows the desired reduction in light based on percentages with the varying degrees and the achieved light reduction. For clarity, this information was plotted, as described in the next section. B. Luminance profiles through time The final luminance profile in Figure 10 was the stopping point due limitations of the overall design of the stimulus box.. Since the luminance around the periphery of the interior dome must be the same as the luminance at the top or center, the goal was to increase the amount of reflecting light to make the two equal. The geometry of the light stimulus box was ultimately the limiting factor in the luminance profile that could be produced with a single point light source. A possible solution to this might to be increase the distance between the reflective box walls and the dome surface, either by using a larger box or a smaller diameter dome. Although the stimulus source that was created in lab does not meet our goal of uniform luminance (+/- 5%), it is the best that could be achieved with the given materials. Degrees 0 10 20 30 40 50 60 70 Average % change 0 to 70 degrees Sum of Squares SS(x)= Original Luminance Profile sc cd s m-2 2790 2730 2310 1790 1380 1190 1070 945 1775 Ideal Percent Change % 66.12 65.38 59.09 47.2 31.52 20.58 11.68 0 Intermediate Final Achieved Luminance Luminance Percent profile Profile Change -2 -2 sc cd s m sc cd s m % 976 952 65.87 1102 941 65.53 1216 973 57.87 1142 965 46.08 923 872 36.81 811 837 29.66 705 781 27 634 745 21.16 938 883 66 47 22 ∑(𝑥 − 𝑥̅ )2 29,135,625 7,358,431 6,295,678 Sum of Squares/Average ∑(𝑥 − 𝑥̅ )2 𝑥̃ 16,414 7,844 7,129 Table 1: Light source calibration data. A comparison between the original luminance profile and the luminance profile with the addition of the star stop. The goal was to decrease the amount of light at every degree interval such that the luminance at that point would be equal to that of the lowest measured value. This decrease in luminance is called the ideal percent change that would be necessary to have uniform luminance. The percent change achieved in the final luminance profile and the ideal percent change necessary for an optimal full field light-flash stimulus have very similar values from 0 to 40 degrees. However at 50 degrees and beyond, the limitations of the light stimulus box design became apparent. A) 3000 sc cd s m-2 2500 StDev/Average = 0.57 2000 Original Luminance Profile 1500 1000 Average 500 0 0 10 20 30 40 50 60 70 Degrees from vertical 3000 B) sc cd s m-2 2500 StDev/Average = 0.29 2000 Intermediate Luminance profile 1500 1000 Average 500 0 0 10 20 30 40 50 60 70 Degrees from vertical C) 3000 sc cd s m-2 2500 StDev/Average = 0.10 2000 1500 Final Luminance Profile 1000 Average 500 0 0 10 20 30 40 50 60 70 Degrees from vertical Figure 10: Luminance profiles. Plotted values are calculated based on (the average of 5 flashes per degree increment.) The standard deviation divided by the average is reported as an indicator of the flatness of the profile. (A) Original luminance profile. (B) Intermediate star stop luminance profile. (C) Final star stop luminance profile. 6. Specific Aim 2- To improve the design and function of the rat-sized meERG contact lens electrode array (CLEAr Lens™), with the goals of increasing the yield of functional electrodes on each lens and improving the quality of the recorded signal. This was accomplished by changing the design of the PMMA base lens and by substantially improving the way in which the assembled CLEAr Lens™ was prepared for recording. 6.1 Methods Prior to an animal recording, the CLEAr Lens™ must be assembled as well as tested to evaluate the number of working channels. The lens assembly consists of the combination of the PMMA base lens, an adhesive double sided tape as well as a cable which connects to the amplifier. Figure 11(B) shows a detailed schematic of the rat base lens as well as a photograph of the assembled rat CLEAr Lens™. The lens assembly steps are described below. - 22 - A) B) Figure 11: (A) Photo of rat lens (B) to-scale schematic of rat lens. A. Lens assembly procedure The lens assembly procedure began by selecting a cable and making sure that the cable was free of dust and debris. A lens that was clear of tape residue was selected and placed into an ultrasonic instrument cleaner for 5 minutes. The lens was then removed and rinsed under distilled water for several seconds. In order to make sure that the lens was completely dry, it was allowed to air dry on a paper towel for 10 minutes. Three 1/8 inch balls of dental wax were then placed equidistant from one another around the inner perimeter of the lens assembly well, see Figure 12. The concave side of the lens was placed down into the lens assembly well assuring that the lens was parallel to the assembly table. The cable was placed onto the metal cable holding shelf with the exposed electrode pad side down and covered with the plastic slip. The red screw was tightened to hold the plastic slip as well as the cable in place. The x, y, z translation knobs and rotation lever were used to adjust the lens such that all the holes were aligned with the electrodes on the cable. Once everything was aligned, the tip of the cable that rests over the lens was pull back and flipped back onto itself such that a small weight could be used to hold in down. A piece of pre-formed tape was removed using forceps and positioned onto the lens such that the holes were all aligned. Using forceps, each precut piece of tape that covered the wells in the lens were removed. Once all the tape from the wells was removed, the weight holding down the cable was carefully lifted and the cable was allowed to fall onto the tape attached to the lens. It was often necessary to use forceps to gently guide the cable back into proper position. Once the cable was aligned, gentle pressure was applied to ensure that the tape made a tight bond. The cable was then removed from the metal cable holding shelf by unscrewing the red screw and lifting the plastic cover. Using forceps, gentle pressure was applied at the side of the lens until the lens was free from the dental wax. Any residual dental wax was removed using a Kimwipe™ and rubbing alcohol. Metal weight that holds flipped cable Rotation lever Assembly well Screw hole Shelf to support cable Figure 12. Photo of the lens assembly table showing the metal cable holding shelf, the assembly well and the metal weight. The assembly well, which is a recess to accept the lens is on a rotation plate which rides on an XYZ translator. B. Revision of Lens Design The voids that are present in the base lens separate the electrode pads from the tear film present on the cornea. These voids need to be filled with a conductive material in order to facilitate a flow of current. Optimum lens fit is critical in order to achieve a high channel yield and minimize cross-talk via a thick tear film between the lens and the cornea. Several modifications were made to the lens design in order to improve on its function. i. Analysis of rat eye size vs age Rat eye curvature measurements were taken twice a week starting at 4 weeks of age in two male rats in order to determine the age at which the lens best fit the rat eye. The measurements were performed using the software DinoCapture and ImageJ. ii. Measurement Procedure using DinoCapture 2.0 version 1.3.2 The animal was first anesthetized and the eye lubricated by applying a drop of saline. The animal was then placed into a stereotactic table and its head was secured into position using the bite bar. A ruler was then placed as close to the eye as possible making sure that it was in the same focal plane as the horizon of the cornea when the camera viewed the eye from the side. Using the "Folder" menu, a folder was created with the appropriate name including the rat id number and the date. This folder needed to be created with DinoCapture in order to save the bitmap images taken by the microscope such that measurements can performed later on. The "Text" option was then used to create a label on the images. The magnification was then set to 1 in the top menu. At this point the position of the ruler was adjusted to make sure it was in the same focal plane as the horizon of the cornea when the camera views the eye from the side. If these were not in the same plane, the depth of the focus on the camera would be different and as a result produce a fuzzy image. By clicking on the Camera icon on the top left corner of the image between 5 and 7 pictures were taken. These pictures were then saved under a subfolder named "Picture" of the folder created at the beginning. In order to make sure that everything was properly saved, the "Folder" menu was used again and the folder containing the correct file name selected. A preview of the images was displayed on the left panel of DinoCapture and one image was selected for analysis. Using the "Calibration" option on the top right of the menu a new calibration profile was created with a corresponding name (rat id, date and image number). At this point the magnification was set to 1 by using the ruler on the image to the known distance of 2 mm that corresponded to the ruler on the screen. By using the “three points arc" on the top menu, three spots on the circumference of cornea were selected and r, the radius of curvature was measured, Figure 13. A total of 3 images per animal were used in calculating the radius of the rat cornea. Figure 13. Photo of eye and ruler captured using DinoCapture camera. Arc drawn for analysis of eye radius shown in white with the calculated radius equal to 2.652 mm. Courtesy of Hadi Tajalli. iii. Measurement Procedure using ImageJ The measurement procedure using ImageJ was very similar to that of the procedure with DinoCapture. The desired jpg image was opened using the ImageJ software. The “Straight” line tool was selected to mark the edge of the hash marks on the ruler and a straight line was drawn from the left edge of one hash mark to the left edge of the next. This was done to set the scale under “Analyze -> Set Scale” in the ImageJ toolbar. In the “Known Distance” box, the distance of the length of the line drawn was entered in mm. The chosen unit of measure then set to be the “Unit of Length.” The “Elliptical” option from the top menu was used to create a circle such that the "w"(width) and "h"(height) of ellipse was equal to diameter of the circle drawn. The "ar" aspect ratio in this case was one. This drawn circle encompassed the entire cornea. Measurements were repeated on three images per animal. iv. Depth of the lens cup The depth of the concave part of the lens was modified in order to improve the contact between the lens and the eye by minimizing the contact between the lens and the eyelids. This was achieved by sanding the eye side of the base lens from 2.97 mm to a variety of depths. The depth that is referred to here is pictured in Figure 14. The 2.16 mm lens fit the curvature of the rats eye the best while the 2.14 mm one was found to be too shallow. The 2.16 mm lens is pictured next to the original 2.97mm lens in Figure 15. A) B) Figure 14: (A) Schematic of the base lens, (B) Photo of base lens. v. Width of the lens voids The voids in the CLEAr Lens™ were typically filled manually by using a polyamide microelectrode filler needle (Microfil 28 guage, MF28G67-5) which had a steep learning curve. The filler needle was slightly smaller than the voids and would break easily, lodging itself in the void in the process. This rendered the CLEAr Lens™ unfit for use and a new lens had to be assembled. Similarly, the use of the filler needle introduced small bubbles that would be trapped in the voids that were difficult to remove. The goal of increasing the width of the voids was to decrease the time and effort it takes to fill the lenses with saline and to increase channel yield. The width of the voids was increased from 0.30 µm to 0.40 µm using a hand drill bit. This was done to all 12 voids in the periphery of the lens (A ring, see Figure 15) and the central void. C. Lens Filling Procedures i. Saline At the beginning of this study, the CLEAr Lens™ was filled with conductive saline manually by using a polyamide microelectrode filler needle (WPI™ Microfil 28 guage, MF28G67-5) and filling 25 voids in order to complete the electrical contact between the corneal surface and the metal electrodes in the lens cable. This involved inserting the tip of the filler needle into the bottom of the void and delivering saline via a hand held syringe all the while slowly withdrawing it from the void. This was a tedious, time consuming process that often yielded less than perfect results due to the filler needle breaking off in the lens voids and trapping air bubbles that were difficult to remove. These air bubbles interfered with the electrical connection, while the metal electrodes were often damaged due to physical scraping by the filler needle. To achieve a higher yield of working electrodes per lens, a better method of filling the lens holes was needed. In order to avoid the manual filling procedure, the use of a vacuum chamber was investigated. Parameters to be optimized included the amount of saline to be place in the lens cup, level of vacuum applied as well as temperature and duration. After a great deal of optimization, the following stranded operating procedure was established. ii. Saline lens filling procedure The rat lens was checked for secure attachment to the cable as well as any foreign debris. The lens was then placed into a Petri dish with the concave side facing up. Using a 1 ml syringe, 3 drops of saline were placed onto the lens. The Petri dish was then placed into the vacuum chamber making sure that the depressurizing value was in the open position and the pressurizing valve was in the closed position. The vacuum was then turned on until the pressure gauge reached 28.5 inHg. When the pressure gauge reached 28.5 inHg, the saline solution was continuously watched for slight changes in opacity due to freezing of the solution which typically started after about 60 seconds. Upon slight change in clarity of the saline, the vacuum pump was shut off and the depressurizing valve was turned to the closed position. If the saline froze, the procedure failed and needed to be repeated. If the procedure was successful, the lens remained in the vacuum chamber for a minimum of 5 minutes. The lens cannot be left in the chamber for a longer period of time due to evaporation of the saline. After 5 minutes had elapsed the air was slowly introduced back into the vacuum by opening the pressurizing valve. Using a microscope, the lens was inspected to see if any bubbles were present in the saline or in the lens voids. If any bubbles were found, the procedure had to be repeated. iii. Saline lens cleaning and storage procedure The lens was first placed into a Petri dish and by using a Kimwipe™, the excess saline was wiped off. Using a 1 ml syringe, 3 drops of distilled water were placed into the lens and the Petri dish was placed into the vacuum chamber. The depressurizing valve was set to the open position while the pressurizing valve was set to the closed position. The vacuum was turned on until the pressure gauge reached 24 inHg. The pressurizing valve was then opened and the air was allowed to slowly enter the vacuum chamber. The depressurizing of the lens and the addition of distilled water was repeated for a total of three time all the while wiping the previously used distilled water in between the cycles. The lens was then removed from the vacuum chamber and the distilled water was wiped off using a Kimwipe™ from the lens. The lens was then stored exposed to air. iv. Poly-Ethylene Glycol (PEG) While the vacuum method had substantially improved the filling procedure as well as the functional yield of electrodes, it was still time consuming and an imperfect process that required practice in order to obtain consistent results. The ideal solution would be to fill the voids in the lens permanently with a clear conductive material that is safe for contact with the cornea. One of the materials that was considered was a hydrogel called poly-ethylene glycol (PEG). This gel is easily made conductive by incorporating free ions such as Na+ and Cl- . The PEG fabrication procedure was optimized by Melanie Kollmer from Richard A. Gemeinhart’s lab. v. PEG fabrication and lens filling procedure Courtesy of Melanie Kollmer, PEG was fabricated by mixing 83 µL of Irgarcure 2959 to 0.817 ml of saline. This mixture was then added to 100 mg of PEGDA (Mw=3400). The rat lens was then checked for secure attachment to the cable as well as any foreign debris. The lens was then placed into a Petri dish with the concave side facing up. Using a 1 ml syringe, 3 drops of PEG were placed onto the lens. The Petri dish was then placed into the vacuum chamber making sure that the depressurizing value was in the open position and the pressurizing valve was in the closed position. The vacuum was then turned on until the pressure gauge reached 28.5 inHg. When the pressure gauge reached 28.5 inHg, the saline solution was continuously watched for slight changes in opacity due to freezing of the solution which typically started after about 60 seconds. Upon slight change in clarity of the saline, the vacuum pump was shut off and the depressurizing valve was turned to the closed position. If the PEG froze, the procedure failed and needed to be repeated. If the procedure was successful, the lens remained in the vacuum chamber for a minimum of 5 minutes with a black cloth covering the chamber in order to minimize the amount of UV light entering the chamber. The lens was checked by opening the pressurizing valve and allowing the air back into the chamber. Using a microscope, the lens was inspected for bubbles. If none were present, the lens was placed under a 360 nm UV light for 40 minutes to polymerize the PEG. If bubbles were present, the filling procedure had to be repeated. Once the PEG polymerized, 4 drops of saline were placed onto the PEG and allowed to sit for 15 minutes to ensure that the PEG was fully hydrated. Once the PEG was fully hydrated, the excess was scraped out of the voids such that the remaining PEG was flush with the curved sides of the lens. vi. PEG lens storage procedure The storage of PEG varied based on whether the lens was to be stored short or long term. If the lens was to be used daily, a drop of saline from a 1 ml syringe placed onto the PEG every day kept the PEG properly hydrated and functional. The addition of a Petri dish cover was used to reduce evaporation. If the lens was to be stored long term, using a 1 ml syringe, 5 drops of distilled water were placed onto the PEG and the excess wiped off with a Kimwipe. The lens was then stored in an air tight chamber. Prior to the use of a lens filled with PEG that was stored for a duration of time, 3 drops of saline from a 1 ml syringe were placed onto the PEG and allowed to rehydrate for 15 minutes. 6.2 Results A. Analysis of rat eye size vs age Rat eye measurements that were taken from two male rats were used to develop an understanding of how rat eye size changes as a function of age. Figure 15 illustrates this compiled data starting at 24 days of age. According to the data, the rat eye would be an ideal fit for the lens at 31 days of age since the lens radius of curvature is 2.5 mm and the radius of curvature of the rats eye at that age is an average of 2.53 mm. Upon testing the lens at this age it was found that the lens was too large to fit properly. This error was attributed to the improper alignment of the rat eye during the eye size measurement procedure as well as the marker placement of the arc calculating software. Based on manual manipulation which consisted of feeling for a secure fit that kept the rat lens on the eye without falling off it was determined that the lens fit first became acceptable at 42 days of age. Based on manual manipulation which consisted of feeling for a secure suction like fit that kept the rat lens on the eye without falling off it was determined that the lens fit best at 42 days of age. Rats that were used for meERG recording were 42 and 49 days of age plus or minus a day for each target eye. y = 1.2812x0.1948 3.0 2.9 Curvature Radius (mm) 2.8 2.7 Measurement difference 2.6 2.5 Lens radius 2.4 2.3 2.2 2.1 2.0 20 25 30 35 40 45 50 55 60 65 70 Age (days) Figure 15: Rat eye radius measurements plotted from the age of 24 to 66 days. Measurements were taken from two male rats with 3-5 days of rest between measurements to reduce stress from repeated anesthesia. According to the measurements done in ImageJ and DinoCapture, the rats eye is a perfect fit for the lens at the age of 31 days. However, upon manual inspection of the lens it was found to best fit at 42 days. Symbols plot measurements from two animals as well as the error between the measured radius and radius of the eye that fits the 5 mm lens. A line was fit to the averaged measurements from two rats and is displayed. This trend line was used to calculate the age at which the lens was to fit. B. Depth of Lens Cup The depth of the lens was modified in order to improve the lens seating on the cornea. By creating a shallower lens, it was no longer necessary to pull back the eye lids of the rat in order to maintain good electrical contact. The photos in Figure 16 show the original and modified lens, 2.16 mm on the left and the original 2.97 mm on the right. Figure 16: Two photos of the modified and original depth of lens at 2.16 mm on the left and the original 2.97 mm on the right. The modification was made using sandpaper and a custom jig to ensure controlled removal of PMMA material. The shallower lens ultimately proved to better fit the curvature of the rats eye. The voids within the lens were widened using a hand-held drill bit. The wider voids can be seen in the central and peripheral rings in both lenses. C. Width of lens voids The increase of the width of the wells did not improve either the time it took to fill the voids or reduce the number of bubbles that were often trapped in the voids during the filling procedure. To the contrary, once the lens was filled and tipped, the probability of saline dripping out was increased due to the increase width of the voids. The wider voids can be seen in Figure 15 located in 12 peripheral (A ring) and one central void. Increasing the width of the voids reduced the likelihood of the filler needle breakage but this was not important once the vacuum filling method was adopted. No difference in impedance was observed between the different widths of voids. D. Lens Filling Procedures The decrease in filling time and an increase in channel yield reached near perfection (24 or 25 working channels) with the vacuum method when compared to the manual filler syringe procedure. The channels that were often found to not work were later determined to be flawed due to an incomplete etching process during cable fabrication. This vacuum filling procedure was later applied to PEG and allowed for a consistent number of working channels as well as a faster experimental setup if the experiments were carried out daily. The major drawback to PEG was the initial filling procedure as well as long term storage. Unless the lens was filled with PEG flawlessly the first time, the lens and cable assembly had to be taken apart to remove the PEG, thereby wasting a cable. The initial stages of perfecting the PEG filling technique produced a number of wasted cables. Long term storage proved to be difficult as certain channels discontinued working upon rehydration. It appeared as though the PEG separated from the gold pads on the cable after long term dehydration. Saline lens storage proved to be easier with the disadvantage of a longer daily setup. Similarly, much of the saline filling procedure depended strongly on visual feedback and experience and often needed to be repeated after bench top testing to confirm channel yield. The channel yield proved to be higher for the saline filled lens due to ability to repeat the filling procedure and test the yield prior to the experiment. On a one time fill basis, the fill success rate was about the same for both materials with 23 to 25 working channels. The need to repeat the filling procedure for both materials would ultimately prove to be a challenge if it was necessary to scale up manufacturing. The vacuum filling technique was adopted for filling the human CLEAr Lens™ and is now standard lab procedure with 100% channel yield. The impedance of channel 77 on the CLEAr Lens™ was measured by a lab mate, Hadi Tajalli and can be seen in Figure 17. Impedance(k Ohm) 200 150 100 50 0 0 200 400 600 800 1000 1200 Frequency (Hz) Figure 17: Impedance of channel 77 on the CLEAr Lens™ performed using saline. Courtesy of Hadi Tajalli. 7. Specific Aim 3- To record the meERG signal from normal rats and to characterize this relatively novel signal with regard to test-retest and inter animal variability and to quantify spatial differences in meERG potentials across the rat cornea. 7.1 Methods Experiments were carried out according to the ACC protocol number 11-154. The rat retina was illuminated using the spatially homogeneous light stimulus source developed in Aim 2, set to the - 40 - strengths listed in Table 2. A aluminum cradle was used to hold the rat steady and position the eye at the center of the light source with the optical axis of the eye at approximately zero degrees to the normal of the source. The subject then received approximately full-field illumination. The recording protocol is developed below. Figure 18 shows a photo of a rat during recording. Filter size (in inches) Flash strength -3.8 (sc cd s m-2) Flash strength -2.8 (sc cd s m-2) 0.0787 1.144 1.614 1.986 2.812 3.455 3.984 0.01 5.8 20 30 73 116 159 n/a n/a n/a n/a n/a n/a 319 Table 2: Light source calibration table with light from filters 0.0787 to 3.894 inches. The power level was increased from level -3.8 to -2.8 on the flash head power pack and the measurement taken at the largest filter size. The stimulus strength of 159 sc cd s m-2 was found to be saturating and was the highest stimulus strength used for rat recordings. The abbreviation n/a indicated that data was not collected at that filter size. A. Recording Protocol The animal recording procedure began by weighing the animals and administering the appropriate amount of anesthetic of Ketamine 100 mg/mL and Xylazine 20 mg/mL under a dim red light. Once the animal was sedated, corneal anesthetic Proparacane 0.5%, and dilators Phenyephrine 2.5% and Tropicamide 1% were administered one minute apart. The ground electrode needle was then placed beneath the skin in the back of the rats neck while the reference electrode was placed in the cheek. The CLEAr LEns™ was then quickly tested for channel yield and placed onto the rat eye. The rat was positioned such that the axis of the right eye was perpendicular to the light stimulus box. Consistency of positioning the animal was based on visual feedback. MCRack was turned on to recording mode and the number of working electrodes was confirmed. If certain electrodes discontinued working, the lens was readjusted on the animal. The smallest filter was placed into the filter slot in the light stimulus box and lowered to the appropriate height from the rats eye. All wires going into the Faraday cage were turned off and or unplugged. Alternating filter sizes was done in the dark with a head light set on red light. Starting with the 0.01 sc cd s m-2, flash strength was increased in the following order; 5.8, 20, 30, 73, 116 and 159 sc cd s m-2 until a total of 7 flash strengths were used. Four flashes at least one minute apart were recorded for each flash strength. A multi channel systems (MEA-60) amplifier was used for recording and the signal sampled at 5 kHz. A typical duration for an experiment was 30 minutes and did not require a booster of anesthetic. Once the recording session was completed successfully, the rat was returned to its cage. Artificial tears were place onto both eyes and the rat was monitored until it woke up. Figure 18: A photograph of an anesthetized rat during a recording with the CLEAr Lens™ in place. Courtesy of Hadi Tajalli. B. Analysis Protocol Data to be used for later analysis was collected using MCRack and later transferred to Excel. Files corresponding to the appropriate series of flash strengths were grouped together, ordered, averaged and the a- and b-wave amplitudes evaluated. A-wave amplitudes were determined by first averaging 50 seconds of the baseline before the flash. Using Excel, the amplitude of the waveform was taken at t = 8.2 ms after the flash. This amplitude was subtracted from the baseline to give the final a-wave amplitude. The b-wave amplitude was determined by finding the maximum value after t = 0 and subtracting the baseline from this value. 7.2 Results A main goal of the NEVL is to research the potential of the meERG to be used as a tool for diagnosis and the monitoring of eye diseases that affect the retina. A large number of rat models of human eye diseases exist today so in order to demonstrate the efficacy and safety of the new CLEAr Lenstm, rats were used. The final Aim of this work was to characterize the novel meERG signal by recording from normally-sighted rats in hope that this normative data will provide a comparative foundation for animal models of retinal dysfunction. A. Typical meERG response A typical meERG response recorded with the CLEAr Lens™ filled with saline can be seen in Figure 19(A). Each meERG waveform is the average of four flashes. Each waveform is offset vertically and horizontally such that is appears in the correct relative position of the corneal electrode from which it was recorded. This type of plot will be referred to as a “position plot” below. The channel yield in this example is relatively high with a few centrally located channels that have a strong stimulus artifact. It is possible to subtract the artifact but for the purposes of demonstrating what a typical flash evoked response looks like as well as a 25 channel yield it was not done for this figure. Figure 19(B) illustrates the channel map, which is the relative location of the recording electrodes on the lens. A) Nasal Superior Inferior B) Temporal Figure 19: (A) Position plot (experiment 20111123-23,24,25,26) where responses to four flashes at 73 sc cd s m-2 were averaged with SNR= 103 dB. Anatomical orientation of the lens is noted in the plot; electrode A8 was at the most superior position. The SNR value was calculated using the following equation 𝑆𝑁𝑅 = 20𝐿𝑜𝑔 10 waveform was offset in order to reflect its actual position on the lens. (B) meERG CLEAr Lens ™ 𝑆𝑖𝑔𝑛𝑎𝑙 𝑁𝑜𝑖𝑠𝑒 . Each electrode map. In order to illustrate the variability of a-wave and b-wave amplitude values, three animals were chosen that had a high channel yield with little artifact. The analysis was performed on the same animals at 6 and 7 weeks of age, and is summarized in Table 3. The mean a-wave and b-wave amplitudes were calculated by averaging all the electrodes. The standard deviations were then divided by the mean and multiplied by one hundred in order to comparison between animals. The symbol N designates the number of working channels that were used to calculate the mean within a particular experiment. The resulting a- and b-wave means are highly varied from one experiment to the next, as is typical for conventional ERG recording. The standard deviations of the a-wave amplitudes are smaller than the standard deviations of the b-wave amplitudes. The normalized standard deviations vary not only from one animal to the next but also between 6 and 7 weeks of age within the same animal. In order to better understand this trend the ratios of the standard deviation divided by the mean were plotted in Figure 20. Experiment date 73 sc cd s m-2 6 weeks A SD/Mean B SD/Mean Experiment Date 7 weeks A SD/Mean B SD/Mean Rat 1 20111123-A N=22 Mean StDev 331 3.2 1.0 1399.7 33.3 2.4 20111129-b N=22 568.9 3.2 5.6 1768.9 22.3 1.3 Rat 2 20110824-b N=24 Mean StDev 243 5.1 2.1 1348.3 188 13.9 20110830-a N=16 206.9 1.0 5.0 1316.9 19.9 1.5 Rat 3 111122 N=23 Mean StDev 440 16 3.6 1550.7 18 1.2 111128 N=22 482.5 4.3 9.0 1504.2 14.5 1.0 Table 3: Mean a- and b-wave amplitudes with all electrodes averaged, stimulus = 73 sc cd s m-2. Three animals were chosen to compare the differences in amplitudes between the animals as well as the differences at week 6 and 7 within one animal. The symbol N designates the number of working channels that were used to calculate the mean within a particular experiment. Standard deviation divided by the mean values have been multiplied by 100. B) Normalized StDev 10 8 6 Rat 1 4 Rat 2 Rat 3 2 15 Normalized StDev A) 0 10 Rat 1 Rat 2 5 Rat 3 0 6 weeks 7 weeks 6 weeks 7 weeks Figure 20: (A) The normalized standard deviation of the a-wave amplitudes from Table 3 (B) The normalized standard deviation of the b-wave amplitudes from Table 3. Three animals were chosen to illustrate the differences in amplitudes as well as at weeks 6 and 7 within one animal. The a-wave amplitudes across all 25 electrodes for 22 experiments were averaged together and their standard deviations plotted in Figure 21 (flash strength 73 sc cd s m-2). The mean was calculated to be 440, StDev = 122. In order to compare the experiments to one another, each experiment was normalized by dividing the standard deviation by its mean, Figure 22. The mean of this ratio was calculated to be 0.04, StDev = 0.02. 700 600 500 µV 400 300 200 100 0 Figure 21: At 73 sc cd s m-2 the a-wave amplitudes of all electrodes for 22 experiments were averaged together and their standard deviations plotted. The mean was calculated to be 440, StDev = 122. Each bar represents an experiment. 0.12 StDev/mean 0.1 0.08 0.06 0.04 0.02 0 Figure 22: The mean divided by the standard deviation of Figure 21 plotted. Each bar represents an experiment and has the same sequence as does Figure 21. Mean = 0.04, StDev = 0.02 B. Inter animal variability Intra-subject data is not always available for comparison, often requiring a clinician to use a database of subjects to determine if the result is within a normal range. The acquisition of a normative data set is critical for the effectiveness of any test. The main goal of the experiments performed in this study was to begin to document the normal range and understand the variability of the inter-animal meERG results such that any measurements taken at a future time will have a normal range to compare to. i. A-wave by ring In order to further understand the basis for the large variances within an experiment, data were grouped by concentric rings formed by the rat CLEAr Lens™ electrodes. This produced evaluation areas at equal radial distances from the center of the cornea and allowed for an increase in sensitivity and in noise reduction in measuring differences between central and peripheral response amplitudes. This approach in grouping of data is utilized by many imaging techniques such as the mfERG (ISCEV, 2012) and while it limits the ability to evaluate asymmetry in the responses, it increases SNR for evaluating differences as a function of radial eccentricity. With this in mind, the mean a-wave amplitudes in the A, C and M rings were normalized to the ring B mean and plotted in Figure 23. The following formulas were employed for calculating the normalized ring averages, 𝐴̅ 𝑁𝑜𝑟𝑚𝑎𝑙𝑖𝑧𝑒𝑑 𝐴 = 𝐵̅ 𝐶̅ 𝑁𝑜𝑟𝑚𝑎𝑙𝑖𝑧𝑒𝑑 𝐶 = 𝐵̅ 𝑁𝑜𝑟𝑚𝑎𝑙𝑖𝑧𝑒𝑑 𝑀 = Equation 1 ̅ 𝑀 𝐵̅ Since the a-wave amplitudes in ring A have the highest potential for variability due to lens misalignment issues, in order to have confidence in the calculated mean value by which the amplitudes would be normalized, the ring B mean was chosen as the common reference value. The ratios for ring A at 6 and 7 weeks consistently range from 1.0 to 1.4 while the ring C has a slightly wider distribution ranging from 0.94 to 1.03. The ratios of Ring A, B and C at 6 weeks of age were calculated and listed in the table next to each figure. The ratios of the central electrode M are based on the least number of experiments as this centrally located electrode was not always functioning during the full course of the experiment. Overall, the a-wave ratios for three rings regardless of age consistently cluster around 1. This is consistent with corneal potentials that are uniform as a function of radial eccentricity. 1.04 1.02 1.02 1 1 Ratio (A/B) Ratio (A/B) 1.04 0.98 0.96 0.94 0.92 Mean 0.99 1.00 0.94 SD 0.02 0.04 0.92 N 12 10 0.96 1.04 1.04 1.02 1.02 1 1 0.98 0.96 0.94 0.92 0.9 Weeks 6 7 Mean 0.99 0.97 0.94 SD 0.03 0.01 0.92 N 12 10 0.96 1.04 1.04 1.02 1.02 1 1 0.98 0.96 0.94 0.92 0.9 7 weeks 0.98 0.9 6 weeks Ratio(M/B) Ratio(M/B) 7 0.9 6 weeks Ratio (C/B) Ratio (C/B) 0.9 Weeks 6 0.98 7 weeks Weeks 6 7 Mean 0.99 1.01 0.96 SD 0.04 0.05 0.94 N 5 10 0.98 0.92 6 weeks 0.9 7 weeks Figure 23: A wave amplitudes from rings A, C and M normalized to ring B and plotted based on age of the animal. Each bar represents an experiment with the table on the right showing the mean and the standard deviation. The symbol N designates the number of animals that were used to calculate the summery statistics. ii. B-wave by ring Ratios for b-wave amplitudes were normalized to ring B and plotted by ring in Figure 24. The ratios of the b-wave amplitudes varied as much within a ring as did the a-wave ratios. The central electrode, M seemed to have less variance for the b-wave amplitude ratios than the a-wave amplitude ratios. Overall, the variance within the ring was consistent between the a- and b- wave amplitudes. 1.04 1.02 1.02 1 1 Weeks 6 7 Mean 0.99 1.00 SD 0.01 0.05 N 10 10 Weeks 6 7 Mean 0.99 1.01 0.96 SD 0.08 0.06 0.94 0.94 N 9 10 0.92 0.92 Ratio(A/B) Ratio(A/B) 1.04 0.98 0.96 0.94 0.92 0.98 0.96 0.94 0.92 0.9 0.9 7 weeks 1.04 1.02 1.02 1 1 Ratio(C/B) 1.04 0.98 0.96 Ratio(M/B) 0.98 0.9 0.9 6 weeks 7 weeks 1.04 1.04 1.02 1.02 1 1 Ratio(M/B) Ratio(C/B) 6 weeks 0.98 0.96 0.94 7 Mean 1.00 1.00 SD 0.04 0.05 N 7 10 0.96 0.94 0.92 0.92 0.9 0.98 Weeks 6 0.9 6 weeks 7 weeks Figure 24: B-wave amplitudes from rings A, C and M normalized to ring B from animals at 6 and 7 weeks. Each bar represents an experiment. The ratios are consistently similar between all animals, clustering around a ratio of 1. C. Spatial Differences The most important feature of the meERG measurement is that it provides information about the spatial distribution of ERG potentials across the cornea. This is the only ERG measurement technique that measures distinct potentials at different locations on the cornea at the same time. The attempt to quantify differences in the spatial distribution of corneal potentials for normally sighted rats might begin to provide information about the health of the retina. In order to achieve this goal, meERG potentials have been analyzed as a function of electrode position. The a-wave amplitudes across all rings at 73.4 sc cd s m2 were calculated by recording and averaging four flashes together per experiment for 18 experiments. Due to the presence of outliers some of the experiments were not used in this analysis. Ratios of each A ring electrode divided by the mean of rings C and M multiplied by 100 were calculated and are plotted in Figures 25 and 26. The following equation defines the ratios: 𝑅𝑎𝑡𝑖𝑜𝐴𝑛 = 𝐴𝑛 𝐶1+𝐶2+𝐶3+𝐶4+𝑀 ( ) 5 𝐴𝑛 = 𝐴 𝑟𝑖𝑛𝑔 𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑑𝑒 𝑥 100 Equation 2 𝐶1−4 = 𝐶 𝑟𝑖𝑛𝑔 𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑑𝑒𝑠 𝑀 = 𝑀 𝑒𝑙𝑒𝑐𝑡𝑟𝑜𝑑𝑒 This ratio was chosen due to a precedent in our lab developed in a study looking to use the meERG for glaucoma detection. The use of the a-wave amplitudes over the b-wave amplitudes were chosen arbitrarily. Values corresponding to the appropriate bin were divided by 216 (18 experiments multiplied by 12 electrodes per experiment) to find the ratios probability. The mean probability ratio across all 18 experiments was 101.0 with a standard deviation of 4.0. Ratios are ultimately a means of comparison and a way of standardizing the results. 0.2 P(Ratio) 0.15 0.1 0.05 0 88 90 92 94 96 98 100 102 104 106 108 110 112 114 116 118 Value of Ratio (Peripheral vs. Central) Figure 25. Probability distribution of a-wave amplitude ratios for 18 experiments at 73.4 sc cd s m-2. The ratios of each A ring electrodes as defined by equation 2. Values corresponding to the appropriate bin were divided by 216 to find the ratios probability. Mean value of ratio= 101.0, SD = 4.0. 0.4 P (Ratio) 0.3 0.2 0.1 0 90 92 94 96 98 100 102 104 106 108 110 A-Wave Amplitude Ratio (Peripheral / Central X 100) Figure 26: For each A-ring electrode position (A1-A12), the average ratio across the 18 experiments was calculated, resulting in 12 averaged ratios. Mean = 100, SD = 1.6. D. Test-retest (same animal) The variability within a data set plays an important role in clinical diagnosis and the ability to determine whether a condition exists or is changing. Therefore, understanding the repeatability of results from a single subject is critical. These results help identify the change that is normal due to growth or noise and differentiate it from a change that might indicate a clinical condition. In order to characterize this test-retest variability, 8 animals were selected and the same ratios as those used to calculate spatial differences were used to evaluate the a-wave amplitudes at 6 and 7 weeks of age. A-wave amplitudes were determined from the average of four flashes at 73 sc cd s m-2. Figure 27 is a series of histograms of the a-wave amplitude ratios at 6 and 7 weeks from 8 different animals. The group of twelve a-wave ratio values obtained in each of the eight animals at six weeks was compared to the corresponding twelve ratio values obtained at seven weeks using a paired Student's t-test. In five of the eight animals, the p values were greater than the criterion value of 0.05, indicating no significant difference between the initial test and the repeated test one week later. Figure 28 is a histogram with all the a-wave amplitude ratios from 8 rats averaged together at 6 and 7 weeks. When the data for the eight animals were combined, the resulting p-value was 0.08. While this value is above the typical criterion value for statistical significance (0.05 > 0.08), a strict interpretation is that there is only an eight percent chance that the six and seven week measurements are not different, or a 92% chance that they are different. This change in the distribution of the amplitudes might be due to lens placement or fit relative to the stimulus source. In order to improve consistency in the future between one experiment and the next, photographs of lens placement will be taken and the angle between the lens and the luminance source will be analyzed. The issue of significant structural changes that might have occurred at the retina was not investigated as this time as it is unlikely to occur over the course of 7 days. Although the origin of the change of the distributions needs to be further investigated it and has been determined to be beyond the scope of this study, the results nonetheless help set the 3.5 3 2.5 2 1.5 1 0.5 0 p = 0.63 animal 2, 6 weeks animal 2, 7 weeks 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 Count foundation for detection of retinal dysfunction at varying stages of development. A-wave amplitude ratios 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 Count 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 Count 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 Count 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 Count 8 6 4 p = 0.0006 2 animal 3, 6 weeks 0 animal 3, 7 weeks A-wave amplitude ratios 3.5 3 2.5 2 1.5 1 0.5 0 p= 0.02 animal 4, 6 weeks animal 4, 7 weeks A-wave amplitude ratios 7 6 5 4 3 2 1 0 p = 0.0006 animal 5, 6 weeks animal 5, 7 weeks A-wave amplitude ratios 3.5 3 2.5 2 1.5 1 0.5 0 p=0.60 animal 6, 6 weeks animal 6, 7 weeks A-wave amplitude ratios 4 Count 3 p=0.75 2 animal 7, 6 weeks 1 animal 7, 7 weeks 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 0 A-wave amplitude ratios 3.5 3 Count 2.5 p=0.37 2 1.5 animal 8, 6 weeks 1 animal 8, 7 weeks 0.5 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 0 A-wave amplitude ratios 5 Count 4 p=0.18 3 2 animal 9, 6 weeks 1 animal 9, 7 weeks 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 0 A-wave amplitude ratios Figure 27: A wave amplitude ratios from 8 animals at 6 and 7 weeks of age in response to a flash strength of 73 sc cd s m-2. The A-wave amplitude ratios were calculated by dividing each peripheral electrode in ring A by the average of the electrodes in rings C and M. Student’s t-Test was used to determine whether the ratio distributions were different from one week to the next. In five out of eight animals, the six and seven week distributions were not significantly different (p < 0.05). 30 25 Count 20 P=0.08 15 6 weeks 10 7 weeks 5 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 0.91 0.92 0.93 0.94 0.95 0.96 0.97 0.98 0.99 1 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.1 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.2 1.21 1.22 0 A-wave amplitude ratios Figure 28: Eight distributions of a-wave amplitude ratios from figure 25 combined in one plot. Stimulus strength = 73 sc cd s m-2. The A-wave amplitude ratios were calculated by dividing each peripheral electrode in ring A by the average of rings C and M. The mean of the 6 week average was found to be 1.04 and 1.01 at seven weeks. The distribution of a-wave ratios is not different, on average across animals, at 6 weeks than it is at 7 weeks. (P = 0.08) 8. Discussion The overall goal of this study was to further the development of a novel ERG measurement technique, the multi-electrode electroretinogram (meERG). This technique is enabled by the contact lens electrode array, CLEAr Lens™ technology developed in the Hetling (UIC) and Williams (UW, Madison) labs. The meERG approach is unique in that it measures corneal ERG potentials at several locations simultaneously, thereby creating a surface potential map of the cornea. This technique is adaptable to a wide variety of visual stimuli currently used in vision science and diagnostic ophthalmology. The main application of the meERG technique will be to detect spatial differences in the response of the retina that are due to eye diseases such as - 60 - glaucoma, retinitis pigmentosa, and diabetic retinopathy. This study focused on fabricating a stimulus source suitable for meERG recording in rats (Specific Aim 1), improving the design and use of the rat-sized CLEAr Lens™ (Specific Aim 2), and recording and characterizing the first meERG signals from rats to establish the first normative data set for this new type of ERG measurement (Specific Aim 3). 8.1 Summary of Results by Specific Aim A. Summary of results: Specific Aim-1 In order to understand the activity of the retina and reveal areas of abnormal response, the spatial distribution of the light stimulus source must be well understood. Since the relationship between the light stimulus source and the activity of the retina is directly linked, fabrication of the stimulus source was performed in hope that we would obtain a level of spatial homogeneity that exceeded the current gold-standard commercial units. The work done to fabricate the full-field, homogeneous stimulus source required a fundamental knowledge of optics and a significant amount of empirical “trial and error” to reach the design goals. In modifying the stimulus source, the percent change from zero to 70 degrees from the vertical was decreased from 66.13% to 22.80%. A measure of uniformity, the sum of squares error between measurements and a flat line (ideal distribution) was also significantly reduced. The geometry of the light stimulus box was ultimately the limiting factor in the luminance profile that could be produced with an overhead light source. It was not possible to further increase the amount of light available at 40 to 70 degrees without increasing the amount of light at 0 to 30 degrees. The periphery of the dome receives less light (which originates directly overhead) and the only way to increase the amount of light available in the periphery is to add a separate light source to that location. A possible solution to this in the future might include increasing the distance between the reflective box walls and the dome surface, either by using a larger box or a smaller diameter dome such that the amount of light reaching the periphery might be increased. Comparison to commercially available units was hindered because the companies that produce those units do not publish or reveal direct measures of uniformity of luminance. However, the final design has proven satisfactory and robust and is in regular use in the NEVL. This system is used in place of a commercial system, and does not by itself contribute anything novel to the field of ERG recording, other than perhaps serving as an example to other laboratories who wish to construct a low-cost source ($2k for our source, compared to at least $10k for a commercial source). B. Summary of results: Specific Aim-2 The rat eye corneal curvature was quantified as a function of age using two male rats to determine the age at which the CLEAr Lens™ would fit best. According to the software ImageJ and DinoCapture the radius of curvature of the rat eye was 2.5 mm at just over four weeks of age. Attempts to record the meERG at this age revealed that the eye was too small to properly fit the lens. This error was attributed to the improper alignment of the rat eye during the measurement procedure as well as the arc calculating software. The lens was found to best fit at 6 weeks of age via manual manipulation which consisted of feeling for a secure fit that kept the lens on the eye without falling off during handling. Other attempts to measure rat corneal curvature as a function of age could not be found in the literature with the most relevant measurements performed by Chauhuri, (1983) where enucleated rat eyes were used to determine corneal radii of curvature. This study focused on measuring the thicknesses and radii of optical compounds as well as determining the reflective indicies of the lens and tissues with the use of a light beam. Similar experiments as those performed by Chauhuri were performed in mice in 1984 by Remtulla S. and Hellett P.E. Neither of these attempts measure the radius of curvature in live animals as a function of time but rather focused on understanding the anatomy and structure of the rodent eye. Design revisions to the rat CLEAr Lens™ were made to improve the lens preparation (filling lens voids to increase channel yield) and to improve lens fit on the rat eye (making the lens cup shallower). The increase in diameter of the lens voids to improve filling proved to be effective when hand-filling the lenses with a syringe, but offered no advantage when filling the lens using the vacuum method. Lens placement and corneal contact were improved by making the lenses shallower, and all lenses currently in use have been modified in this way. The primary contribution under this aim was the development of the protocol for using a vacuum chamber to fill the lenses. This method offered substantial advantages over hand filling in terms of time required, reduced damage to the CLEAr Lens™ components, and increased channel yield. The next step in this line of improvements will be to permanently fill the lens holes with a conductive, transparent, biocompatible material and thus eliminate most of the lens preparation procedure altogether (lenses still need to be cleaned before and after use). Filling the lens holes with PEG was explored extensively in collaboration with the Gemeinhart lab (UIC), and while recording quality and channel yield were comparable to lenses filled with saline, storage proved to be a problem. To store the PEG in a hydrated state proved unacceptable because of the effects of the long-term hydrated environment on other lens components (adhesive tape). To store the PEG in a semi-dehydrated state required it to be re-hydrated prior to use, which proved to be unreliable in terms of maintaining channel yield. The solution to this problem will require a different lens assembly adhesive material, or exploration of other candidate conductive materials, such as conductive silicone. This will be an active area of development for future students in the NEVL. C. Summary of results: Specific Aim-3 The main contribution of this work was the recording and analysis of the first significant set of meERG data ever obtained. The first account of recording corneal potentials at various locations was by Sundmark in 1959 who used a contact glass fitted with nine electrodes; Sundmark was able to record potential differences across the retina in human subjects. The most recent recordings were done by Holland and Herr in rabbit eyes in 1964. The purpose of Holland and Herrs study was to determine whether electroretinographic changes were present in healthy and post photocoagulated retina. The development of the meERG as described in this thesis has proven to be a direct way of recording as many as 25 waveforms simultaneously. A number of investigators have modeled the ERG source and the resultant corneal potentials, and have described the potential use of such recorded potentials in the study of retinal function. The most significant of these is the work by Job et al. 1999, who concluded that different retinal locations contribute differently to local corneal potentials. Cringle et al. 1989 patented a method of using simultaneously recorded scleral potentials in diagnosis, but their patent did not describe a method of analysis of the recorded potentials or finding a relationship between localized retinal damage and changes in eye surface recordings. An important part of evaluating any new technology or method with potential diagnostic application is to compare it to existing methods that might provide similar information. As the meERG technique has the potential to provide information about spatial differences in retinal function, we here focus on comparing meERG to multi-focal electroretinography (mfERG), which is the only ERG technique that attempts to generate a functional map of the retina. The meERG has several advantages over the mfERG such as its short test time and the ability to probe the entire retina. The meERG is independent of movement, fixation and positioning errors such as those present in the mfERG. (ISCEV, 2011) The meERG waveforms directly reflect the function of the retina, requiring minimal signal processing. Since the meERG uses a Ganzfeld stimulus, there are less restrictions in the stimulus type which can range from standard scotopic flashes to paired-flash protocols. Although the potential disadvantages of the meERG over the mfERG include lower resolution and less sensitivity it is likely that the two techniques may be complimentary in combining the high spatial resolution of the central retina in the mfERG with the information on the peripheral function of the meERG. 9. Cited Literature Cited Literature Chaudhuri A, Hallett P, Parker J. Aspheric curvatures, refractive indices and chromatic aberration for the rat eye. Vision Research 1983; 23(12):1351-1363 Cringle SJ, Alder VA, Brown MJ, Yu DY. Effect of sclera recording location on ERG amplitude. Current Eye Research 1987; 6(9):1109-1114 Cringle SJ. Electroretinogram Apparatus. U.S. Patent # 4,874,237; 1989 Gotch F. The time relations of the photoelectric changes on the eyeball of the frog. J Physiol. 1903;29:388–416 Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol. 1933;77:207–239 He B & Wu D (1999) Laplacian electrocardiography. Crit Rev Biomed Eng 27(3-5):285-338 Hennessy M, Vaegan. Amplitude scaling relationships of Burian-Allen, gold foil and Dawson, Trick and Litzkow electrodes. Documenta Ophthalmologica 1995; 89(3):235-248 Holland M. G. & Herr N (1964) The electroretinographic potential field. Localization of retinal lesions. American Journal of Opthalmology. 57:639-45. Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM. ISCEV standard for clinical multifocal electroretinography (mfERG). Doc Ophthalmo 2012 Feb;124(1):1-13 - 66 - Job HM, Keating D, Evans AL, Parks S (1999) Three-dimensional electromagnetic model of the human eye: advances toward the optimization of electroretinographic signal detection. Med Biol Eng Comput 37:710-719 Kooijman, A. Light distribution on the retina of a wide-angle theoretical eye, J. Opt. Soc. Am. 73, 1544-1550 (1983) Kooijman, A. and Witmer F. Ganzfield light distribution on the retina of human and rabbit eyes: calculations and in vitro measurements, J. Opt. Soc. Am. A 3, 2116-2120 (1986) Lamb, T.D., Pugh Jr. E.N. (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol 449:719–758pmid:1326052. Masland RH (2001) The fundamental plan of the retina. Nature Neurosci 4:877-886 McAllan A, Sinn J, Aylward GW. The effect of gold foil electrode position on the electroretinogram in human subjects. Vision Research 1989; 29(9):1085-1087 Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. Anatomical Distribution of Rods and Cones Remtulla S, Hallett P. A schematic eye for the mouse, and comparisons with the rat. Vision Research 1985; 25(1):21-31 Sundmark E. The contact glass in human electroretinography. Acta Ophthalmalogica Supplement 1959; 52:3-40 Sutter E, Tran D. The field topography of ERG components in man-I. The photopic luminance response. Vision Research 1992; 32(1):433-446 Webvision (2012) http://webvision.med.utah.edu/ 10. Appendix 10.1 Standard operating procedures A. Lens Assembly procedure 1. Select a cable and ensure the cable is free of dust and debris. 2. Select a lens that is clear of tape residue as well as saline. Place lens into an ultrasonic instrument cleaner for 5 minutes. 3. Remove and rinse the lens under distilled water for several seconds and pat dry. 4. Confirm that the wells within the lens are dry and clean. 5. Place three 1/8 in balls of dental wax equidistant from one another around the inner perimeter of the lens assembly well. Figure 12. 6. Place the lens concave side down into the lens assembly well assuring that the lens is parallel to the assembly table. 7. Place the cable onto the metal cable holding shelf, exposed electrode pad side down and cover it with the plastic slip and make sure the red screw is on tight. Figure 12 8. Use the x, y, z translation knobs and rotation lever to adjust the lens such that all the holes are aligned with the electrodes on the cable. 9. Once everything is aligned, pull back the tip of the cable that rests over the lens and flip it back onto itself such that a small weight can be placed to hold in down. Figure 12 10. Using forceps, remove a piece of pre-formed tape and position it onto the lens such that the holes are all aligned. 11. Using forceps remove each precut piece of tape that covers the wells in the lens. 12. Once all the tape from the wells has been removed, carefully remove the weight holding down the cable and allow the cable to fall onto the tape attached to the lens. 13. It may be necessary to use forceps to gently guide the cable back into its original position. 14. Once the cable is aligned, gently push down on to ensure that the tape bonds to it. 15. It is now safe to remove the cable from the metal cable holding shelf by unscrewing the red screw and lifting the plastic cover. 16. Using forceps, push at the lens from the side until the lens is free from the dental wax. 17. Remove access dental wax using a Kimwipe and rubbing alcohol. B. Measurement Procedure using DinoCapture 2.0 version 1.3.2 1. Anesthetize the animal and lubricate the eye by applying one drop of saline. 2. Place the animal in the stereotactic table and secure its head position using the bite bar. 3. Place a ruler as close to the eye as possible making sure that it is in the same focal plane as the horizon of the cornea when the camera views the eye from the side.. - 68 - 4. Using the "Folder" menu, create a folder with appropriate name including Rat # and Date. This folder needs to be created via DinoCapture in order to save the bitmap images taken by the microscope and measurements can be done later. 5. Use "Text" option to create a label on images, also set the magnification to 1 in the top menu. 6. Make sure the ruler us in the same focal plane as the horizon of the cornea when the camera views the eye from the side. If these are not in the same plane the depth of focus will be different for each and produce a fuzzy image. 7. By clicking on the Camera icon on the top left corner of the image take as many as 5-7 pictures. These pictures will be saved under a subfolder named "Picture" of the folder created at the beginning. 8. To open the folder use the "Folder" menu again and select the folder containing the pictures you have taken. Preview of your images will be displayed on the left panel of DinoCapture and select one for analysis. 9. Use "Calibration" options on the top right of the menu and create a new calibration profile and name it corresponding with Rat#, Date and Picture#. Make sure magnification is set to 1 and set (using the ruler on the image) the known distance of 2 mm on the image and finish the calibration. This part is similar to set the scale in Image J software. 10. Use “three points arc" on the top menu to point three spot on the circumference of cornea and measure the r (Radius of curvature) 11. Capture 3 images per animal and repeat the measurements on each image. C. Measurement Procedure using ImageJ 1. Open the jpg image taken by Dino Capture with ImageJ software 2. Select the *Straight* line tool. Choose a hash mark on the ruler and draw a straight line from the left edge of that hash mark to the left edge of a different hash mark. Next go to “Analyze -> Set Scale” under the ImageJ toolbar. In the “Known Distance” box, enter the length the line across the ruler is in mm, and set the “Unit of Length” to mm. 3. Use "Elliptical" option from top menu to create a circle so "w"(width) and "h"(height) of elliptic would be equal to diameter of the circle you draw. In this case "ar" aspect ratio will be one. This circle should encompass the cornea. 4. Capture 3 images per animal and repeat the measurements on each image. D. Saline lens filling procedure 1. Ensure the rat lens is free of foreign debris and is securely attached to the cable. 2. 3. 4. 5. Place the lens into a Petri dish with concave side facing up. Using a 1 ml syringe place 3 drops of saline onto the lens. Place Petri dish into the vacuum chamber. Close chamber door. Ensure the depressurizing valve is in the open position and the pressurizing valve is in the closed position. Turn on the vacuum until the vacuum pressure gauge reaches 28.5 inHg. 6. Once 28.5 inHg is achieved: continuously watch the saline solution for slight changes in opacity due to freezing which starts around 60 seconds. Upon slight change in clarity of saline, shut off vacuum pump and turn the depressurizing valve to the closed position. 7. If saline freezes, procedure has failed. Dispose of used saline and restart process from step #3. 8. Lens must remain in the sealed vacuum chamber for a minimum of 5 minutes. If left in longer, ensure that the saline does not evaporate. 9. Opening the pressurizing valve and allowing the air back into the vacuum chamber. 10. Using a microscope; inspect the lens. If bubbles are present in the saline or in lens wells it will be necessary to repeat the procedure. E. Saline lens cleaning and storage procedure 1. Place the lens in a petri dish and using a Kimwipe wipe off the excess saline. 2. Using a 1ml syringe, place 3 drops of distilled water into the lens and place in vacuum. 3. Close chamber door. Ensure the depressurizing valve is in the open position and the pressurizing valve is in the closed position. Turn on the vacuum until the vacuum pressure gauge reaches 26 inHg. 4. Open the pressurizing valve and allow the air back into the vacuum chamber. 5. Repeat steps #2 through #4 for a total of three times all the while wiping the previously used distilled water from the lens using a kimwipe. 6. Remove the lens from the vacuum chamber and wipe off all of the distilled water from the lens. 7. Store the lens exposed to air. F. PEG lens filling procedure 1. 2. 3. 4. Ensure the rat lens is free of foreign debris and is securely attached to the cable. Place the lens into a Petri dish with concave side facing up. Using a 1 ml syringe place 3 drops of PEG onto the lens. Place petri dish into the vacuum chamber. 5. Close chamber door. Ensure the depressurizing valve is in the open position and the pressurizing valve is in the closed position. Turn on the vacuum until the vacuum pressure gauge reaches 28.5 in Hg. 6. Once 28.5 in Hg is achieved: continuously watch the PEG for slight changes in opacity due to freezing which starts around 60 seconds. Upon slight change in clarity, shut off vacuum pump and turn depressurizing valve to the closed position. 7. If PEG freezes procedure has failed, restart process from step #3. 8. Lens must remain in the sealed vacuum chamber for a minimum of 5 minutes. If left in longer, ensure that the PEG does not evaporate. During this time cover the vacuum chamber with a black cloth to minimize the amount of UV light entering the chamber. 9. Check lens by opening the pressurizing valve and allowing the air back into the vacuum chamber. 10. Using a microscope; inspect the lens. If bubbles are present in the PEG or in lens wells it will be necessary to repeat the procedure. 11. Once there are no more bubbles, place the lens under a 360 nm UV light. Place the light as close to the lens as possible by resting the light on the edges of the petri dish. 12. Allow the PEG to polymerize for 40 minutes. 13. Once the PEG has polymerized, turn off the UV light and place 4 drops of saline into the well with the PEG. 14. Cover the lens with the cover to the petri dish and allow to sit for 15 minutes to ensure that the PEG is fully hydrated. 15. Once the PEG is fully hydrated, scrape the excess out of the well. The goal is to cut the PEG such that it is flush with the curved sides of the lens. G. PEG fabrication procedure 1. To make the PEGDA hydrogel add 83µL of Irgarcure 2959 to 0.817 ml of saline. 2. Add the mixture to 100 mg of PEGDA (Mw=3400). Courtesy of Melanie Kollmer. H. PEG lens storage procedure 1. If the lens will be used daily, simply place one drop of saline from a 1 ml syringe onto the PEG every day to make sure it stays hydrated. 2. Place a small petri dish cover to reduce evaporation. 3. If the lens needs to be stored long term, using a 1 ml syringe, place 5 drops of distilled water onto the PEG filled lens and wipe off the excess with a Kimwipe. 4. Store the lens in an air tight chamber. 5. Prior to using a lens that has been stored long term, place 3 drops of saline from a 1ml syringe and allow the PEG to fully rehydrate for 15 minutes. I. Recording Protocol 1. Animals were anesthetized under a dim red light using Ketamine and Xylazine. 2. Corneal anesthetics of Proparacane, Phenyephrine and Tropicamide were administered one minute apart. 3. The ground electrode needle was place in the back of the rats neck while the reference electrode was placed in the cheek. 4. The CLEAr LEnstm was tested for channel yield and placed onto the rats eye. The rat was positioned such that the axis of the right eye was perpendicular to the light stimulus box. Consistency of positioning the animal was based on visual feedback. 5. MCRack was turned on to recording mode and the number of working electrodes was confirmed. If certain electrodes discontinued working, the lens was readjusted on the animal. 6. The smallest filter was placed into the filter slot in the light stimulus box and lowered to the appropriate height from the rats eye. 7. All wires going into the Faraday cage were turned off and or unplugged. 8. Alternating filter sizes was done in the dark with a head light set on red light. 9. Starting with the 0.01 sc cd s m-2, flash strength was increased in the following order; 5.8, 20, 30, 73, 116 and 159 sc cd s m-2 until a total of 7 flash strengths were used. 10. Four flashes at least one minute apart were recorded for each flash strength. 11. An ALA amplifier was used for recording and the signal sampled at 5kHz. 12. A typical duration for an experiments was on average 30 minutes and did not require an anesthetic booster. 13. Once the recording session was completed successfully, the rat was returned to its cage. Eye drops were place onto both eyes and the rat was monitored until it woke up. J. Analysis Protocol 1. Data was collected using MCRack and transferred to excel. 2. Files corresponding to the appropriate series of flash strengths were grouped together, ordered, averaged and the a and b-wave amplitudes measured. 3. A-wave amplitudes were determined by first averaging 50 seconds of the baseline before the flash. 4. Using excel, the amplitude of the waveform was taken at t=8.2 ms after the flash. This amplitude was subtracted from the baseline to give the final a-wave amplitude. 5. The b-wave amplitude was determine by finding the max value after t=0 and subtracting the baseline from this value. VITA NAME: Yelena Krakova EDUCATION: B.S., Biological Sciences, University of Illinois at Chicago, 2005 M.S., Bioengineering, University of Illinois at Chicago 2012