Melting ice with a good dissolvable salt, a bad dissolvable salt and a
advertisement

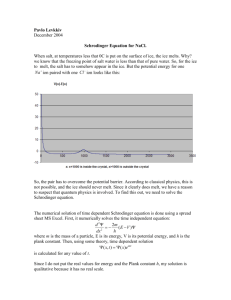
Melting ice with a good dissolvable salt, a bad dissolvable salt and a reactive salt Kan24_tim_tim Summary of inquiry In the winter the roads in Holland are often covered in ice, this endangers the drivers because they can slip away easily and (might) crash. To avoid this, salt is spread out over the roads in order to keep ice from forming and the formed ice to melt as fast as possible. We tested which salt melts the ice fastest by laying salts with different characteristics over ice and check which one melted the ice fastest. The outcome of our test is useful for the government to decide which salt to use. From our tests it appeared that the dissolvable salt melts the ice the quickest. Introduction The goal of our experiment is to see what kind of salt melts ice fastest. Knowing which salt melts ice fastest is practical for the melting of snow and ice on the roads. Based on the fact that a good dissolvable salt when dissolved in water, gives the water a lower melting point, we believe that the ice with the good dissolvable salt will melt fastest. Unfortunately not all salts dissolve in water, some of them do not dissolve in water and some of them even decompose in water, causing the initial salt to fall apart and become other substances. The bad dissolvable and decomposing salts will therefore not dissolve in water, so the melting point of water stays the same. Method & Materials -filter -ice cubes, four times two ice cubes of 13 grams each -NaCl, 0,5 grams -Mg3(PO4)2, 2,25 grams -(NH4)2CO3, 0,85 grams -stopwatch -measuring glass (100mL) -funnels We put the filters onto the funnels and put the ice cubes in them. After this we carefully dropped the different salts on the ice cubes and started measuring the time with the stopwatch. We also had a pair of ice cubes with no salt on them at all. We made sure that biggest part of the surface of the ice cube was covered with the salt. The amount of grams of each salt was calculated with the molar masses of the salts so that each salt had about the same amount of molecules on the ice cubes. Every ten minutes we checked the amount of water that had dropped into the measuring glass and noted it for each salt until all the ice had melted, when an ice cube was almost melted we watched so we knew exactly the time it was completely melted. Results The amount of water that was melted and had gone through the filter into the measuring glass is shown as a function of the time in table 1. Figure 1 30 Tabel 1 Mg3(PO4)2 (NH4)2CO3 0 0 0 No salt 0 1 mL 0 mL 1 mL 0 mL 4 mL 1 mL 4 mL 2 mL 12 mL 18 mL 21 mL 23 mL 28 mL 5 mL 11 mL 6 mL 25 20 Formed water 15 (mL) 10 5 0 NaCl 10 mL 18 mL 16 mL 21 mL 11 mL 15 mL 19 mL 23 mL 19 mL 22 mL 24 mL 25,5 mL 28 mL 25 mL 26 mL 24 mL 28 mL 26 mL 26 mL 24,5 mL 28 mL 26 mL 26 mL 26 mL Tabel 1: Melted water in function of time. The different salts we used are stated in the top row, underneath that the amount of water that has melted into the measuring glass is stated after periods of 10 minutes. Mg3(PO4)2 (NH4)2CO3 0 min 10 min 20 min 30 min 40 min 50 min 60 min 1h 12 min 1h 14 min 1h 17 min 1h 20 min 0 min 10 min 20 min 30 min 40 min 50 min 60 min 1h 12 min 1h 14 min 1h 17 min 1h 20 min NaCl No Salt Time Figure 1: Formed water in function of time, graph. On the Y-axes is the formed water from the different salts in mL. On the X-axes is the time it took to form this amount of water in the measuring glass. Conclusion & Discussion The ice melted fastest with the good dissolvable salt on its surface, our hypothesis was correct. We have come up with 3 theories why the good dissolvable salt melts the ice so fast. Theory 1. Dissolvable salts are known for, when dissolved in water, lowering the melting point of the water. The salt could have the same effect on the ice, lowering its melting point and therefore speeding up the melting process. Theory 2. Another characteristic of salts is that they absorb water. When the ice cube with the salt on it melts, the liquid water that is formed will be absorbed by the salt, making it impossible for the formed water to evaporate. Evaporation of water costs energy and therefore heat. This heat is now not spilled on the evaporation and so the melting process is speeded up. Theory 3. Water molecules are polar. Salts are electrically charged , so the water will be attracted by the salt because of the difference in electrical charge. Because of this attraction the water molecules in the salt that are already a little bit melted are sucked out of the ice because of the difference of the molecules in charge. Giving it more surface area in the ice for the outer temperature to melt it faster. These theories are ordered in the likeliness of the event actually happening. The salt that decomposes in water was the second fastest in melting the ice. The decomposing salt decomposes into NH3, CO2 and H2O. The first 2 are gases and the water is liquid. We think that in the beginning on the ice it had the same effect as the good dissolvable salt because there wasn’t enough liquid water to make it decompose, after the ice had melted a little, it decomposed and the ice melted just like normal ice, but because the salt had made gaps in the surface of the ice cube there was more surface area for the outer temperature to melt the ice. The bad dissolvable ice didn’t do much accept absorbing some of the water formed, which might make it easier for the ice to melt. However the bad dissolvable ice might have also worked a bit against the melting process for it isolated the ice keeping the ice from warming up by the surrounding temperature. Noticeable was that we got 26 mL of water from every set up and from the NaCl even 28 mL of filtrate while initially we had put 25 mL of water in the ice used in the set up. To explain this we think that we might have measured the amount of water we put into the ice cubes wrong, or there had formed some extra ice on top of the ice cubes in the freezer, or the salt that had gone through the filter with the melted water had given the filtrate some extra volume. What was also interesting was that during the blanco test it did not take much longer for the ice to completely melt than with a salt added. This can be explained as follows. As you can see in our results, in the beginning of the melting process the ice with the decomposing or dissolving salt had melted a lot more than the ice in the blanco test. It was only in the end that the blanco test came closer to the other set ups. This could be so because the water that had melted away in the tests with salts had some salt dissolved in it and therefore there was less salt on the ice to uphold the fast melting process. In the case of the decomposing salt, some of the salt had decomposed after a period of time also leaving less salt to melt the water faster. Sources BINAS