acquired hemolytic anemia - Journal of Evidence Based Medicine
advertisement

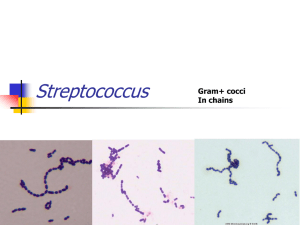
ORIGINAL ARTICLE ACQUIRED HEMOLYTIC ANEMIA Veda P1, Srinivasamurthy V2, Pushpalatha K3 HOW TO CITE THIS ARTICLE: Veda P, Srinivasamurthy V, Pushpalatha K. ”Acquired Hemolytic Anemia”. Journal of Evidence based Medicine and Healthcare; Volume 2, Issue 18, May 04, 2015; Page: 2699-2706. ABSTRACT: Acquired hemolytic anemia is a group of disorders in which premature destruction of red cells is triggered by extrinsic factors. In this study, we have evaluated 38 cases of acquired hemolytic anemia. Immune hemolysis accounted for 52.6% (n=20) and fragmentation hemolysis 44.7% (n=17) of acquired hemolytic anemia. Autoimmune hemolytic anemia (AIHA) and microangiopathic hemolytic anemia (MHA) surfaced as the two most frequent causes accounting for 44.7% (n=17) and 39.5% (n=15) of acquired hemolysis. Erythrocyte morphology gives valuable clues concerning the cause of hemolysis. Spherocytes were observed in 94% (n=16) cases of immune hemolytic anemia. Schistocytes were observed in all cases of microangiopathic hemolytic anemia (MHA). In addition to schistocytes, microspherocytes were seen in 40% of MHA. Precise identification of spherocytes, schistocytes and microspherocytes is very important as it gives valuable information regarding the cause of acquired hemolysis. KEYWORDS: Immune hemolysis, microangiopathic hemolytic anemia (MHA), spherocytes, schistocytes, microspherocytes. INTRODUCTION: Acquired hemolytic anemia represents a group of disorders in which premature destruction of the red cells is triggered by extrinsic factors unlike congenital hemolytic anemia which results from intrinsic red cell defects. Generally, acquired hemolytic anemia can be classified as immune hemolytic anemia and non-immune hemolytic anemia.(1) Immune hemolytic anemia (IHA) is a condition in which IgG and/or IgM antibodies bind to RBC surface and initiate RBC destruction via the reticuloendothelial system and complement system. IHA is classified into autoimmune, alloimmune and drug induced hemolysis. Non-immune hemolytic anemias result from mechanical trauma, oxidative damage, infections, drugs, toxins, PNH and other causes. Mechanical damage to the erythrocytes is caused by excessive shearing forces on red cells. It can also result from fibrin strands on the endothelium of small vessels as seen in microangiopathic hemolytic anemia (MHA).(2,3) Diagnosis of acquired hemolytic anemia requires a careful evaluation of clinical history, biochemical, serological and hematological workup. In this context, complete blood counts and morphology of blood smear can yield valuable information which can guide further course of action. Precise identification of spherocytes, schistocytes, microspherocytes, and irregularly contracted cells plays a vital role in determining the cause of hemolysis.(4) Thorough assessment of erythrocyte morphology can offer information that no automated instrument can yet give. MATERIALS AND METHODS: This study was conducted at ESI PGIMSR for a period of one year between 2012- 2013. Patients who had clinical and laboratory evidence of hemolysis were included in the study. Patients with G6PD deficiency, Heriditary spherocytosis, Hemoglobinopathies and other congenital causes were excluded. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2699 ORIGINAL ARTICLE All pts underwent the following investigations- Complete blood counts (CBC), reticulocyte count, blood smear, lactate dehydrogenase (LDH), liver function tests, renal function tests, serum haptoglobin and Coomb’s test (direct and indirect). Complete clinical details with particular attention to disorders associated with immune hemolytic anemia (IHA), microangiopathic hemolytic anemia (MAH) and drug history were recorded. The diagnosis of immune hemolytic anemia was based on a positive Direct antiglobulin test (DAT).(3) Gel cards with polyspecific antihuman globulin and complement were used to screen the patients. Presence of schistocytes and/ or microspherocytes was taken as evidence of RBC fragmentation.(5) MAH was diagnosed when fragmentation hemolysis was accompanied by thrombocytopenia. Thrombocytopenia was defined as a platelet count less than 1.5x109/L.(6) Fluorescent antinuclear antibody test was used as a screening test for SLE. Prothrombin time, Activated partial thromboplastin time and D-dimer assays were performed to rule out DIC. RESULTS: We encountered 38 cases of acquired hemolytic anemias in this study. Immune hemolysis accounted for 52.6% (n=20) and fragmentation hemolysis 44.7% (n=17) of acquired hemolytic anemia (Table 1). The two most frequent causes of acquired hemolytic anemia were AIHA (n=17) and MHA (n=15). The hematological features of AIHA and MHA are summarized in Table 2. Oxidative hemolysis (n=1) was an uncommon cause of acquired hemolysis Of the 20 cases of IHA, 3 cases were related to alloimmunization (2 cases of hemolytic disease in newborns and one case of delayed transfusion reaction). The remaining 17 cases were categorized as autoimmune hemolytic anemia (AIHA). Of the 17 cases diagnosed as AIHA, 58. 8% (n=10) had serological and/or clinical evidence of underlying disorders. They were identified as SLE (n=5), Rheumatoid arthritis (n=1), pulmonary tuberculosis (n=1), HIV (n=1), Mycoplasma infection (n=1), Small lymphocytic lymphoma (n=1). These 10 cases were classified as secondary AIHA. The remaining 7 cases (41.2%) which did not have any underlying disorders were regarded as primary AIHA (Table 1). Fragmentation hemolysis was evidenced in 17 cases. In two of these cases the cause of fragmentation was identified as severe aortic stenosis and prosthetic heart valve. These two cases were classified as cardiac hemolysis and both had normal platelet counts. Rest of the 15 cases showed varying degrees of thrombocytopenia in addition to erythrocyte fragmentation. These 15 cases were regarded as microangiopathic hemolytic anemia (MHA). Platelet count in these cases ranged from 0.05- 1.2 x109/L and LDH level was elevated in 14 cases (94%). A thorough clinical and laboratory workup was performed in all these 15 cases of MAH to identify the primary cause. Preeclampsia accounted for 20% (n=3) of MHA. Other causes included occurred in SLE (n=1), DIC (n=2), HIV infection (n=1), vacuities (n=1) and disseminated malignancy (n=2). Of the remaining 5 cases, a diagnosis of thrombocytopenic purpura (TTP) was made in 3 cases and Hemolytic uremic syndrome (HUS) in 2 cases based on clinical and laboratory evaluation. Erythrocyte morphology was carefully evaluated in all cases of acquired hemolytic anemia. Spherocytes were identified in 90% (n=18) cases of IHA (Fig. 1). Red cell agglutinates were observed in only one case of cold agglutinin AIHA, which was secondary to mycoplasma infection. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2700 ORIGINAL ARTICLE We encountered one case of chronic AIHA without spherocytes in the blood smear. This patient had thrombocytopenia and secondary folic acid deficiency. His platelet counts improved after correction of nutritional deficiency. Thrombocytopenia in this case was attributed to folic acid deficiency. Thrombocytopenia was also seen in two cases of AIHA secondary to SLE. These two cases were thought to represent Evan’s syndrome.(7) Schistocytes were observed in all 15 cases of MHA, being numerous in (80%) 12 cases (fig 2). 6 cases of MHA (40%) displayed microspherocytes in addition to schistocytes. Spherocytes were also seen in 2 cases (13.3%) of MHA, but were accompanied by schistocytes in both cases. Blister cells and irregularly contracted cells were seen in the case of oxidative hemolysis. DISCUSSION: The present study demonstrated that AIHA and MHA were the most frequent causes of acquired hemolytic anemia. 58.8% of AIHA were secondary to underlying diseases, SLE being the most frequent. Other studies in India have reported the frequency of secondary AIHA as 77% and 29%.(8,9) Baek et al. have observed that majority of patients with primary AIHA were found to have SLE during a followup of two years.(10) In the present study, fragmentation hemolysis represented the most frequent form of non-immune acquired hemolytic anemia. MHA accounted for 88% (n= 15) of fragmentation hemolysis. Cardiac hemolysis was rare, observed in only two cases. Hemolysis caused by damaged heart valves and prosthetic valves have often been reported.(11,12) Microangiopathic hemolytic anemia is a Coombs-negative intravascular hemolytic anemia, with schistocytes in the peripheral smear.(13,14) The term thrombotic microangiopathy (TMA) has been used to describe multiple disease entities characterized by MHA, thrombocytopenia, and organ injury that result from microthrombi in capillaries and arterioles.(14,15) TMA includes two main entities: thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). The primary differentiating factor between HUS and TTP is the presence of significant renal impairment in HUS. A TMA syndrome can also be observed in association with pregnancy, disseminated cancer and chemotherapy, human immunodeficiency (HIV) infection, malignant hypertension, autoimmune disorders, Disseminated intravascular coagulation (DIC), vasculitis, certain drugs and following hematopoietic stem cell transplantation.(6,14,15) In the setting of MHA, a thorough clinical and laboratory workup is warranted to arrive at a precise diagnosis. In our study, MHA resulted from a variety of causes. Preeclampsia, TTP and HUS were the frequent causes. It is important to distinguish preeclampsia related MHA from TTP/HUS and HELLP since the treatment modalities are different.(16) We also encountered a case of HIV infection, where TTP was the first manifestation of HIV. The association of HIV and TTP has been reported.(6,17) Since IHA and MHA surfaced as the most frequent causes of acquired hemolysis, their hematological and biochemical features were compared (Table 2). Both showed female predilection. The mean platelet count in MAH was 0.46 /L, which was much lower than 2.3/L in IHA. Spherocytes were observed in 88% of immune hemolysis and only 13% of non-immune hemolytic process. Spherocytes most often indicate either hereditary spherocytosis or autoimmune haemolytic anemia. It has been established that spherocytes in the blood smear of J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2701 ORIGINAL ARTICLE IHA are erythrocytes that are coated with IgG and/ or complement that have been damaged by phagocytic cells.(18) Presence of spherocytes in the blood smear warrants a DAT.(5) Schistocytes were observed in 86.7% of MHA and only 11.7% of immune hemolysis. Schistocytes are circulating fragments of erythrocytes or amputated erythrocytes from which fragments have been lost.(19) Schistocytes should be identified by specific morphological criteria. Schistocytes are always smaller than intact red cells. They can have the shape of fragments with sharp angles and straight borders, small crescents, helmet cells, keratocytes, or microspherocytes. Schistocytes result from mechanical fragmentation of erythrocytes caused by fibrin strands on the endothelial surface and/or excess of turbulence of blood. They are usually absent or very rare in blood films of healthy individuals.(19) Schistocytes, if accompanied by thrombocytopenia are a strong cytomorphological indicator of TMA. In such a context, HUS/ TTP should always be considered because of the urgent need for institution plasma exchange therapy.(13) However, schistocytes are not pathognomonic of TMA as they are also seen in other conditions like structural abnormalities of the heart and great vessels or a malfunctioning prosthetic valve.(11,12) Unlike TMA, these cases do not have thrombocytopenia.(19) Schistocyte-like cells may appear in non-TMA-related genetic or acquired RBC disorders. In these conditions, schistocytes occur in a backdrop of severe anisopoikilocytosis and do not essentially reflect erythrocyte fragmentation. However, when schistocytes represent an isolated or dominant abnormality, it is a strong indicator of MHA.(19,20) The presence of an extraordinarily high LDH in the presence of microangiopathic hemolysis and thrombocytopenia should prompt a diagnosis of TMA.(6) Microspherocytes are small spherocytes, which also have a smooth outline and lack central pallor. However, unlike spherocytes, they represent erythrocyte fragments which have respherized in circulation. Hence, microspherocytes are also regarded as spheroschistocytes. They indicate that red cell fragmentation has occurred. In the context of MHA, microspherocytes are invariably accompanied by schistocytes.(4) In the present study, microspherocytes were seen in 40% of MAH and were accompanied by schistocyte in all cases. Microspherocytes should be distinguished from irregularly contracted cells which also appear hyperchromatic but have an irregular outline. They result from oxidative damage and are usually accompanied by blister cells and bite cells.(4) CONCLUSIONS: AIHA and MHA are the most frequent forms of acquired hemolytic anemia. Precise identification of spherocytes, microspherocytes and schistocytes is very important in discerning the cause of hemolysis. In the context of acquired hemolysis, spherocytes point towards IHA. Schistocytes and microspherocytes indicate fragmentation hemolysis. Schistocytes if accompanied by thrombocytopenia should prompt a diagnosis of MHA. Even in the era of automation, morphology of blood smear offers extremely valuable information that no advanced equipment can offer. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2702 ORIGINAL ARTICLE ACQUIRED HEMOLYTIC ANEMIA (n=38) Cause of Acquired Hemolysis 1. Immune hemolysis (52.6%) i. Autoimmune Primary (41.2%) Secondary (58.8%) ii. Alloimmune Hemolytic disease of newborn Transfusion reaction 2. Fragmentation hemolysis (44.7%) n 20 17 7 10 3 2 1 17 Microangiopathic hemolysis Cardiac hemolysis 3. Oxidative hemolysis (2.6 %) 15 2 1 Drug induced Table 1: Showing the various types of acquired hemolytic anemia encountered in the study 1 Table 2: The summary of epidemiological and hematological findings in acquired hemolytic anemia is shown. The findings in AIHA and MHA, the two most common causes of acquired hemolysis are compared. (numbers in parenthesis indicate range). Patient characteristics Age (years) F: M Hb (g/dl) Hematocrit (%) MCV (fl) Reticulocytes (%) 43.6 5.2: 1 7.9 23.8 91.1 AIHA (n=17) 38 7: 1 6.9 (2.3 – 11.6) 21.5 97 (88- 117) MAH (n=15) 49 2.1: 1 8.8 (1.8- 12.3) 27 88 (67- 99) 4.1 3.9 (1.8- 23) 4.3 (0.6-28.5) 7.6 (3.6- 14.7) 2.3 (0.8- 6.3) 8.9 (2.1- 21.6) 0.46 (0.05- 1.2) 45.6% 31% Total WBC (x 109 / L) 8.3 9 Platelets (x 10 / L) 1.38 Total bilirubin 38.3 % > 1.3 mg/ dl LDH >400 IU/L 82.3 % Spherocytes 62.5% microspherocytes 28% Schistocytes 46.8% 70.6 % 94% (n=16) 12% (n=2) 6% (n=1) Table 2 94% 13 % (n=2) 40% (n=6) 100% (n=15) J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2703 ORIGINAL ARTICLE Fig. 1: Blood smear from a case of AIHA showing numerous spherocytes. Polychromasia and nucleated red cells are seen. An myelocyte is also seen reflecting a shift to left. Hb, reticulocyte and platelets were 7.3g/dl, 1.7x 109/L and 12% respectively. Fig. 1 Fig. 2: Blood smear from a patient diagnosed as TTP showing numerous schistocytes. Platelet count was 0. 05 x109/L. Polychromasia and nucleated red cells are evident. A closer look shows the characteristic angular edges and straight border of schistocytes (inset). Fig. 2 Fig. 3: blood smear from a case of HUS showing numerous microspherocytes with severe thrombocytopenia. Shistocytes are also seen in the smear indicating red cell fragmentation. Fig. 3 J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2704 ORIGINAL ARTICLE REFERENCES: 1. Kelton JG, Chan HH, Heddle NM, Whittaker S. Acquired hemolytic anemia. In: Wickramasinghe SN and McCullough J (eds). In: Blood and Bone Marrow Pathology, Edinburgh, Scotland: Churchill Livingston, Elsevier Science Limited, 2003: 185-202. 2. Han-Mou Tsai. Thrombotic thrombocytopenic purpura, Hemolytic Uremic syndrome and related disorders (1314-1324) in Wintrobes Clinical Hematology-Volume 2. 12 th edition, Published in 2009 at Philadelphia USA, by Lippincotts Williams and Wilkins. 3. Zeerleder S. Autoimmune haemolytic anaemia – a practical guide to cope with a diagnostic and therapeutic challenge. The Netherlands Journal of Medicine. 2011; 69 (4): 177-184. 4. Barbara J. Bain. Spherocytes and irregularly contracted cells. Basic Haematology. Haematology Watch, Vol 3, Issue 1. 5. Bain BJ. Morphology in the diagnosis of red cell disorders. Hematology 2005; 10 (1): 178 /181. 6. Thompson CE, Lloyd E, Damon LE, Ries CA, Linker CA. Thrombotic Microangiopathies in the 1980s: Clinical Features, Response to Treatment, and the Impact of the Human Immunodeficiency Virus Epidemic. Blood 1992; l80 (8): 1890-1895. 7. Michel M, Chanet V, Dechartres A et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009; 114 (15): 31673172. 8. Alwar V, Devi AM Shanthala, S Sitalakshmi, Karuna R K. Clinical Patterns and Hematological Spectrum in Autoimmune Hemolytic Anemia. J Lab Physicians. 2010; 2 (1): 17–20. 9. Choudhry VP, Passi GR, Pati HP. Clinico-hematological spectrum of auto-immune hemolytic anemia: an Indian experience. J Assoc Physicians India. 1996; 44 (2): 112-4. 10. SW Baek, MW lee, HW Ryu et al. Clinical fearures and outcomes of autoimmune hemolytic anemia: a retrospective analysis of 32 cases. Korean J of Hematology. 2011; 46 (2): 111117. 11. Tsuji A, Tanabe M, Onishi K, Kitamura T, Okinaka T, Ito M, Isaka N, Nakano T. Intravascular hemolysis in aortic stenosis. Intern Med. 2004; 43 (10): 935-8. 12. Shapira Y, Vaturi M, Sagie A. Hemolysis associated with prosthetic heart valves: a review. Cardiol Rev. 2009; 17 (3): 121-4. 13. Tefferi A, Elliott MA. Schistocytes on the Peripheral Blood Smear. Mayo Clin Proc. 2004; 79 (6): 809. 14. Halevy D, Radhakrishnan J, Markowitz et al. Thrombotic microangiopathies. Crit Care Clin. 2002; 18: 309-320. 15. Coppo P, Veyradier A. Thrombotic Microangiopathies: Towards a Pathophysiology-Based Classification. Cardiovascular & Haematological Disorders-Drug Targets, 2009; 9: 36-50. 16. Stella CL, Dacus J, Guzman E, et al. The diagnostic dilemma of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome in the obstetric triage and emergency department: lessons from 4 tertiary hospitals. Am J Obstet Gynecol 2009; 200: 381. e1-381. e6. 17. Ahmed S, R K Siddiqui, A K Siddiqui, S A Zaidi, J Cervia. HIV associated thrombotic microangiopathy. Postgrad Med J 2002; 78: 520–525. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2705 ORIGINAL ARTICLE 18. Sir John Dacie. The immune hemolytic anemias- a century of exciting progress in understanding. British Journal of Haematology, 2001, 114, 770-785. 19. Zini G, D’Onofrio G, Briggs C, Erber W et al. ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. International Journal of Laboratory Hematology 2012; 34 (2): 107-116. 20. J. F. Lesesve, Salignac S, Lecompte T. Diagnosis of mechanical hemolytic anemias: contribution of complete blood count. Annals of clinical biology. 2001; 59 (5): 551-8. AUTHORS: 1. Veda P. 2. Srinivasamurthy V. 3. Pushpalatha K. PARTICULARS OF CONTRIBUTORS: 1. Assistant Professor, Department of Pathology, ESIC PGIMSR, Rajajinagar, Bangalore. 2. Professor & HOD, Department of Pathology, ESIC PGIMSR, Rajajinagar, Bangalore. 3. Professor & HOD, Department of Paediatrics, ESIC PGIMSR, Rajajinagar, Bangalore. NAME ADDRESS EMAIL ID OF THE CORRESPONDING AUTHOR: Dr. Veda P, # 10, Sri Devi Krupa, 1st Main, 1st Block, R.T. Nagar, Bangalore-10. E-mail: vedalallu@yahoo.com Date Date Date Date of of of of Submission: 19/04/2015. Peer Review: 20/04/2015. Acceptance: 23/04/2015. Publishing: 29/04/2015. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 2/Issue 18/May 04, 2015 Page 2706