Chemoselective Transfer Hydrogenation of Aldehydes and Ketones
advertisement

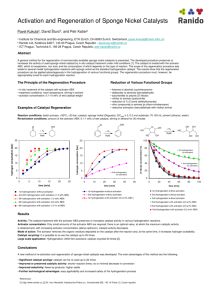
Supplementary material Chemoselective Transfer Hydrogenation of Aldehydes and Ketones with a Heterogeneous Iridium Catalyst in Water Zhi Wang, Lei Huang, Longfei Geng, Rizhi Chen, Weihong Xing, Yong Wang and Jun Huang* * State Key Laboratory of Materials-Oriented Chemical Engineering College of Chemistry and Chemical Engineering, Nanjing Tech University, Nanjing, Jiangsu 210009 (P. R. China) E-mail: junhuang@njtech.edu.cn Experimental section General Methods and Reagents Reagents were purchased from Aladdin Reagent Company, Sigma-Aldrich Company and Alfa-Aesar Company, and used without further purification. The GC (Shimadzu GC-2014C) analysis was carried out with capillary column RTX-5(Cat. #10254) (RESTEK Corp. ). The size and morphology of the nanoparticles were observed by a JEOL-2010 HRTEM operating with 200 kV. Ir content of the catalysts were measured by inductively coupled plasma (ICP) on Optima 2000DV. Thermogravimetry (TG) analysis was carried out with a STA409 instrument under dry nitrogen at a heating rate of 20 oC/min. The optimization of the reaction conditions Table 1S. Ir@CN catalyzed transfer hydrogenation of 4-methyl benzaldehydea 0.5 mol% Ir@CN HCOOH O OH Entry HCOOH (eq) NaOH (eq) Conv. (%) Sel. (%) pH value b 1 2 3 4 5 6 7 8 9 5 4 3.5 3 2 1.5 3.5 3.5 3.5 0 0 0 0 0 0 1 2 3 96 95 95 90 84 73 95 94 75 79 84 87 89 92 97 94 >99 >99 2.01 2.15 2.22 2.30 2.41 2.52 3.15 3.66 4.42 Supplementary material 10 a Reaction 3.5 3.5 30 >99 6.9 conditions: 4-methyl benzaldehyde, 1 mmol, water 3.5 mL, catalyst Ir@CN (48 mg, 0.5% Ir), 100 oC, 16 h; conversion and selectivity were determined by GC analysis; b the pH value were the starting pH value. The transfer hydrogenation of 4-methyl benzaldehyde was chosen as a template reaction to optimize reaction conditions. The amounts of formic acid and NaOH were optimized as table 1S, and 3.5 equivalent HCOOH with 2.0 equivalent NaOH was used as the suitable reductive. The reaction time, reaction temperature, the amount of catalyst were also optimized for the thansfer hydrogenation of 4-methyl benzaldehyde, and the results are summarized in Table 2S. And the continuous measurements of conversion and selectivity as a function of reaction time were presented in Fig. 1S. Table 2S. Ir@CN catalyzed transfer hydrogenation 4-methyl benzaldehyde under various conditionsa 3.5 eq HCOOH 3.5 ml water 2 eq NaOH OH O a Reaction Entry Catalyst (Ir%) T (℃) Time (h) Yield (%) 1 2 3 4 5 6 7 0.5 0.5 0.5 0.5 0.5 0.25 0.1 100 100 100 60 80 100 100 16 18 20 18 18 18 18 94 99 99 67 88 68 39 conditions: 4-methyl benzaldehyde, 1 mmol; water, 3.5 mL; HCOOH, 3.5 eq.; NaOH 2.0 eq.; catalyst Ir@CN. conversion and yield were determined by GC analysis. Supplementary material Fig. 1S Continuous measurements of conversion and selectivity as a function of reaction time