Practical Ecology booklet
advertisement

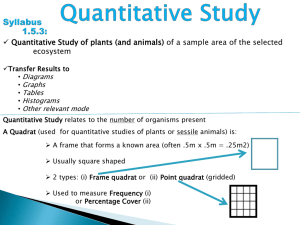
Practical Ecology Pupil Workbook IB Environmental Systems and Societies Name: INTRODUCTION TO SAMPLING Ecology is often referred to as the "study of distribution and abundance". This being true, we would often like to know how many of a certain organism are in a certain place, or at a certain time. Information on the abundance of an organism, or group of organisms is fundamental to most questions in ecology. However, we can rarely do a complete census of the organisms in the area of interest because of limitations to time or research funds. Therefore, we usually have to estimate the abundance of organisms by sampling them, or counting a subset of the population of interest. For example, suppose you wanted to know how many ants there were in the park in Chapultepec. It would take a lifetime to count them all, but you could estimate their abundance by counting all the ants in carefully chosen smaller areas throughout the park. RANDOM SAMPLING USING QUADRATS Random sampling is usually carried out when the area under study is fairly uniform, very large, and or there is limited time available. When using random sampling techniques, large numbers of samples/records are taken from different positions within the habitat. A quadrat is most often used for this type of sampling. The frame is placed on the ground and the animals, and/ or plants inside it are counted. This is done many times at different points within the habitat to give a large number of different samples. In the simplest form of random sampling, the quadrat is thrown to fall at ‘random’ within the site. However, this is usually unsatisfactory because a personal element inevitably enters into the throwing and it is not truly random. True randomness is an important element in ecology, because statistics are widely used to process the results of sampling. Many of the common statistical techniques used are only valid on data that is truly randomly collected. This technique is also only possible if quadrats of small size are being used. It would be impossible to throw anything larger than a 1m2 quadrat and even this might pose problems. Within habitats such as woodlands or scrub areas, it is also often not possible to physically lay quadrat frames down, because tree trunks and shrubs get in the way. In this case, an area the same size as the quadrat has to be measured out instead and the corners marked to indicate the quadrat area to be sampled. A better method of random sampling is to map/mark out your area to be sampled with tape or rope. Using a pair of random numbers you can locate a position within the sampling area to collect your data. The random numbers can be pulled from a set of numbers in a hat, come from random number tables, or be generated by a calculator or computer. The two numbers are used as coordinates to locate a sampling position within the area. The first random number gives the position on the first tape and the second random number gives the position on the second tape. USING A QUADRAT TO MEASURE ABUNDANCE OF A SPECIES The usual sampling unit is a quadrat. Quadrats normally consist of a square frame, the most frequently used size being 0.25m2 (see picture below). The purpose of using a quadrat is to enable comparable samples to be obtained from areas of consistent size and shape. Each square within this quadrat represents 4% of the quadrat. IN Decide which borders are going to count as ‘in’ and which will count as ‘out’. Any organisms that fall on the lines deemed as being ‘out’ should not be counted. OUT IN OUT MEASURING DENSITY OF A SPECIES 1. Calculate the percent that each square of your quadrat is worth (show your working below) area = ………………………….m2 2. Name the species you will be investigating: …………………………. 3. Mark off the area to be sampled using chalk, tape etc. It is recommended that the smallest area to be sampled is 25m x 25m 4. Use a random number generator to generate 10 sets of coordinates. These coordinates will tell you where to place your quadrat. You can do this by picking numbers out of a hat. 1st random number 7 2nd Random number 18 25 Area to be sampled marked off Bottom left hand corner of quadrat laid down where two numbers intersect 18 0 0 7 25 5. Count the number of individuals in your quadrat – remember to use the ‘in’ and ‘out’ rule! Quadrat number 1 2 3 4 5 Number of individuals a) Calculate the mean number of individuals per quadrat …………………………. per …………………………. m2 (this is the area of your quadrat) b) Now calculate how many quadrat areas would fit into your sample area (sample ÷ area) …………………………. 6. Multiply the your answer for mean number of individuals by your answer for 5.b) This will give you an estimated number of individuals in your whole sample area. …………………………. in …………………………. m2 7. Now complete your data processing by calculating how many individuals are found per m 2 (divide the first part of your answer to question 6 by the second part of your answer to question 6) …………………………. per m2 MEAURING PERCENTAGE COVER OF A SPECIES In many plant species e.g. grasses it is very difficult to count the number of individual plants so calculating density is not possible. Count the number of squares within the quadrat that the species completely covers. Then count the squares that are only partially covered. Use this information to estimate the total number of squares that would be completely covered. 8. Calculate the percentage of each square in your quadrat (100 ÷ no. of squares) Each square = ………………………….% 9. Name the species you will be investigating: …………………………. 10. Mark off the area to be sampled using chalk, tape etc. It is recommended that the smallest area to be sampled is 25m x 25m 11. Use a random number generator to generate 10 sets of coordinates. These coordinates will tell you where to place your quadrat. You can do this by picking numbers out of a hat. 1st random number 7 2nd Random number 18 25 Area to be sampled marked off Bottom left hand corner of quadrat laid down where two numbers intersect 18 0 0 7 25 12. Count the number of squares within the quadrat that the species completely covers. Then count the squares that are only partially covered. Use this information to estimate the total number of squares that would be completely covered. Fill in the ‘Number of Squares column’ Quadrat number 1 2 3 4 5 Number of squares Percentage cover (%) ACFOR scale 13. Multiply the figure in the ‘Number of squares’ column by your answer to Q8. Write your answer in the ‘Percentage cover’ column. Repeat for all 10 quadrats. The ACFOR Scale ACFOR is an acronym for a simple, somewhat subjective scale used to describe species abundance within a given area. It is normally used within a sampling quadrat to indicate how many organisms there are in a particular habitat when it would not be practical to count them all. The ACFOR scale is as follows: A = ABUNDANT (greater than / equal to 51%) C = COMMON (26-50%) F = FREQUENT (12-25%) O = OCCASIONAL (6-11%) R = RARE (6% to single individual) 14. Us e the information above to determine the abundance for each quadrat and write this into the ‘ACFOR Scale’ column. 15. Calculate the mean percentage cover per quadrat …………………………. per …………………………. m2 (this is the area of your quadrat) 16. Now calculate how many quadrat areas would fit into your sample area (sample ÷ area) …………………………. 17. Multiply your answer for mean percentage cover by your answer for 15.) This will give you an estimated mean percentage cover in your whole sample area. …………………………. in …………………………. m2 18. Now complete your data processing by calculating percentage cover per m2 (divide the first part of your answer to question 17 by the second part of your answer to question 17) …………………………. per m2 How would you describe the percentage cover per m2 based on the ACFOR scale? ………………………….…………………………. MEASURING ABIOTIC FACTORS Measuring the abiotic factors in each sample site is very important. The abundance and diversity of species is dependent on the environmental conditions in an ecosystem. When sampling an ecosystem: Make 2 readings with each sensor at the central position from the quadrat and at 2 different levels in each quadrat: ground level and 1 m height. Central position for sensors readings: X Abiotic factor reading positions in each quadrat 19. Measure the following abiotic factors using the sensors/instruments available Abiotic factor Light intensity Temperature Soil moisture Soil pH Reading at ground level (±unit) Reading at 1m (±unit) MEASURING SPECIES DIVERSITY 20. Identify three different species in your site. You don’t have to know their name. You can take a picture or draw them. For the purposes of this exercise, draw three different plants in the boxes below. Species A Species B Species C 21. Mark off the area to be sampled using chalk, tape etc. It is recommended that the smallest area to be sampled is 25m x 25m 22. Use a random number generator to generate 5 sets of coordinates. These coordinates will tell you where to place your quadrat. You can do this by picking numbers out of a hat. 1st random number 7 2nd Random number 18 25 0 7 25 Count the number of individuals of each species in each quadrat. Species A B C Bottom left hand corner of quadrat laid down where two numbers intersect 18 0 23. Area to be sampled marked off 1 Number of individuals in each quadrat 2 3 4 5 24. Calculate Simpson’s Diversity Index for each quadrat Quadrat 1: Species A B C Number of Individuals (n) n(n-1) Total N D Ʃn(n-1) N ( N 1) 142 141 3.21 n(n 1) 6246 Quadrat 2: Species A B C Number of Individuals (n) n(n-1) Total N D Ʃn(n-1) N ( N 1) 142 141 3.21 n(n 1) 6246 Quadrat 3: Species A B C Number of Individuals (n) n(n-1) Total N D N ( N 1) 142 141 3.21 n(n 1) 6246 Ʃn(n-1) Quadrat 4: Species A B C Number of Individuals (n) n(n-1) Total N D Ʃn(n-1) N ( N 1) 142 141 3.21 n(n 1) 6246 Quadrat 5: Species A B C Number of Individuals (n) n(n-1) Total N D Ʃn(n-1) N ( N 1) 142 141 3.21 n(n 1) 6246 25. Calculate the mean Simpson’s Diversity Index for the site. Fill in the table below with your answers from question 24. Quadrat Simpson’s Diversity Index 1 2 3 4 5 Mean SYSTEMATIC SAMPLING USING QUADRATS Systematic sampling is used when the sampling area includes an environmental gradient; where physical conditions change within the locality (for example a sloped hillside where factors like wind speed may increase as you move up the hill). Sampling of saltmarshes and sand dunes requires this approach, as the environmental gradient in these habitats is very distinct. Systematic sampling involves having structure to your method in order to obtain data in a series, rather than at random. A transect is required to systematically sample through an environmental gradient. Transects can be of many types, the two most common being line transects (sampling along a line, e.g. a series of points along a tape measure) and belt transects(sampling within an area, e.g. a series of quadrats). Belt transects can be of two main distinctions. Continuous belt transects sample in an unbroken manner through the entire environmental gradient (for example by turning a quadrat end-over-end). Interrupted belt transects leave some areas un-sampled (e.g. placing a quadrat at 10 metre intervals). Of these two types of transects, the interrupted belt transect is the one most frequently used for conducting fieldwork within large areas e.g. saltmarshes or sand dunes. Line transect Continous belt transect Interrupted belt transect INTERRUPTED BELT TRANSECT 26. Identify three different species in your site. You don’t have to know their name. You can take a picture or draw them. For the purposes of this exercise, draw three different plants in the boxes below. Species A Species B Species C 27. Mark of a line using string/ chalk of 25m. Choose a site that shows a gradual environmental change e.g. going up a slope or changing from cleared ground to overgrown or between 2 different habitats. 28. Lay down your quadrat every 5m and measure the percentage cover of that particular species in your quadrat. Quadrat 29. Complete the data table below Quadrat position (m) 0 5 10 15 20 25 Species A Percentage cover Species B Species C 30. Plot a kite graph showing distribution of species along your transect. Kite graphs are ideal for representing distributional data (e.g. abundance along an environmental gradient). They are elongated figures drawn along a baseline. Each kite represents changes in species abundance across a landscape. The abundance can be calculated from the kite width. They often involve plots for more than one species; this makes them good for highlighting probable differences in habitat preferences between species. The central line for each diagram has a value of 0. The ‘kite’ is then drawn symmetrically both above and below the line to represent your data. Drawing a kite diagram The x axes of the kite diagrams are drawn to the same scale as the slope profile and the y axis plotted as a percentage with 50% above the x axis and 50% below the x axis. Vegetation is then plotted at the sampling distance along the x axis. Half of the percentage is plotted above the x axis and half is plotted below it. The plotted points on both sides of the x axis are then joined together with a straight line to produce kite/diamond shapes and these can then be shaded. CALCULATING THE ABUNDANCE OF MOVING ORGANISMS The Lincoln Index n1 = number of animals first marked and released n2 = number of animals captured in the second sample m = number of marked animals in the second sample N = total population The Lincoln Index makes several assumptions that must be met if the estimate is to be accurate. These assumptions are: A.) The population of organisms must be closed, no immigration or emigration. B.) The time between samples must be small compared to the life span of the organism. C.) The marked organisms mix completely with the rest of the population in the time between sampling. D.) Marks do not harm organisms or disappear between samples. Lincoln Index model experiment 1.) The plastic bag represents the habitat of your model population. Add 5 big handfuls of dry beans to the habitat. The beans represent your organisms in your habitat. NOTE: Do not try to count the exact number of beans until the end of the experiment. 2.) Remove a small handful of beans from the model habitat. This handful will be your first sample. The sample should be at least 20 beans but less than half the total population. 3.) Using the paint, mark all organisms in this first population. Mark them well enough to be easily identified if recaptured. Count the beans and record this number as n1 for trial in the table on the next page. Let the marks dry on the beans. Number of beans in first sample = ………………………….. 4.) Place the beans from your first sample (n1) back into the habitat. Mix them well. 5.) Without looking, one member of the group should remove another handful of beans. The sample size should be about the same as the original. Count the total number of beans in the second capture. This is your n2 value for trial 1. Notice that some of the beans will have the marking from the first capture. Count these organisms and record this number as m for trial 1. Number of beans in second sample = ……………………………. Number of marked beans in second sample = ………………………….. 6.) Return the organisms to their habitat. Mix well. 7.) Repeat steps 5 and 6 two more times giving you a total of three trials or three recaptures. Complete the table. 8.) Count the actual number of beans. Actual population size = ………………………….. Trial 1 n1 Trial 2 n1 Trial 3 n1 n2 n2 n2 m m m Lincoln Index Lincoln Index Lincoln Index What is the difference between the estimated population size in each trial and the actual population size? Trial 1 = ………………………….. Trial 2 = ………………………….. Trial 3 = …………………………..