Shawn Haynes - parkerorganicchemistrysp2014
advertisement

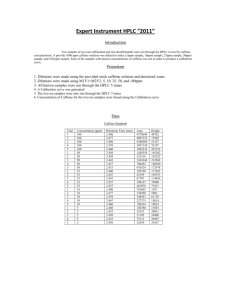
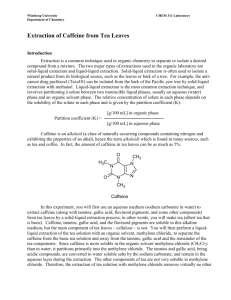
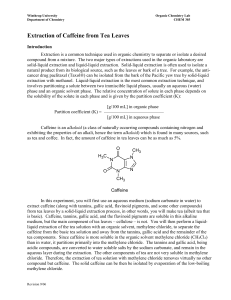
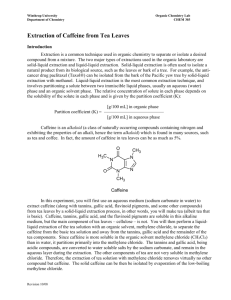
Extraction of Caffeine from Tea Objective: Too efficiently extract the caffeine from tea leaves using the solvent, methylene chloride. Background: Caffeine was first isolated by a German chemist by the name of Fredrich Ferdinand Runge in 1819, although it had been consumed by humans for many years, in different plants, teas, and coffee. Caffeine is the worlds most consumed psychoactive substance. Caffeine is very compatible with many systems within the body because its structure is very similar to some neurotransmitters, and it also has a pH of 6.9, almost neutral, which is close to the pH of blood. The feeling of greater alertness or lack of fatigue and drowsiness is due to its CNS, central nervous system, stimulus properties. Since caffeine is naturally able to cross over the blood brain barrier the chemical is able to bind to and block activity from certain receptors that, if activated, would decrease neural activity and slow blood flow to smooth muscle. Because these inhibitory mechanisms are not able to occur, the subject has the sensation of feeling energetic and alert. Like with any stimulant, regular consumers of substances that contain a significant amount caffeine do develop a very high tolerance to the affects that caffeine has on the nervous system. Caffeine is also commonly found in drugs that are administered to premature newborns or given during neonatal intensive care. Results: Preparing Tea Mixture: Initially, the tea bag was weighed and the weight of the actual tea leaves within the bag was calculated. Table 1 displays all of the initial weights and measurements. Then the tea bag was submerged and simmered in 20mL of water for 15 minutes. After separating the tea mixture into two centrifuge tubes, sodium carbonate was added to each tube. Next them methylene chloride was added. These two components are critical in the process of caffeine extraction. The sodium carbonate serves as the substance that binds to the water within the tea mixture. Caffeine is much more soluble in methylene chloride than water, so the methylene chloride serves to separate the caffeine from the remaining organic molecules within the tea mixture. Table 1 (Initial weights and measurements) Total weight of tea bag and Tea bag alone = .23g contents = 2.53g Sodium carbonate (NaCO3) .5g (mol wt. 84.01g/mol) Methylene Chloride 3mL (mol wt. 84.93g/mol) (CH2Cl2) Weight of tea leaves = 2.3g .006 moles .04 moles Extraction and Drying: After all initial components had been added and mixed thoroughly the two tubes containing the mixtures were capped and put into a centrifuge, where they were spun until two clearly defined layers appeared. Using a Pasteur pipet, the bottom clear layer containing methylene chloride and caffeine was extracted, put into a clean 25ml Erlenmeyer flask, and the process was repeated for a second time. Since there will be a small amount of water that is retained within the clear solution, granular anhydrous sodium sulfate is added to the solution in order to absorb this remaining water. The process is complete when small clumps begin to form. The remaining solution will have very few contaminants and be almost pure methylene chloride and caffeine. Evaporation: The solution was removed from the flask containing the granular anhydrous sodium sulfate drying agent, and placed in a clean, pre-weighed Erlenmeyer flask. This solution was heated over a warm water bath in order to evaporate the methylene chloride of the solution and the dried crystalline structures that remained were caffeine. Table 2 shows our weights and percent recovery. Table 2 (Flask weights and caffeine percent recovery) Weight of clean, dry flask Weight of flask and crude Crude caffeine weight = = 24.235g caffeine = 24.262g 0.027g Percent Recovery (0.027g)/ (2.3g) = 0.0117% X100% = 1.17% recovery Conclusion: Caffeine was successfully isolated and extracted from tea leaves using a technique that required methylene chloride. Since caffeine is present in tea leaves at about 4%, a percent recovery of 1.17% shows that more than ¼ of the caffeine was extracted. Discussions: First I must express my disappointment over the fact that the sublimation apparatus that the school had was unreliable, and that successful sublimation was able to be seen, due to leaks in the vacuum and hoses. I believe the sublimation process would have been very interesting to witness and watch after seeing the results from the evaporation technique. Visualizing this experiment and each step involved, helped me to find a greater understanding of why certain substances are used at specific times. Such as when granular anhydrous sodium sulfate is used to absorb the remaining water, and why methylene chloride is used as the solvent opposed to water. These properties and principles became very clear during this experiment. Overall the experiment went very well, although it was very difficult, almost impossible, to extract the bottom layer of clear solution without transferring any tea particles or residue over into the clean flask. I thought that the percent recovery that was obtained was very good. It showed that we had recovered over one fourth of the original caffeine from the tea leaves. I think that using a different technique, other than the Pasteur pipet to extract the methylene chloride/caffeine solution would help to increase our percent recovery.