Lab 8. Photosynthesis
advertisement

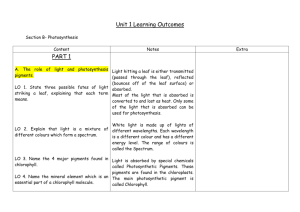
Chapter 10. Photosynthesis: UNDERSTANDING PHOTOSYNTHESIS Student Learning Outcome s At the completion of this exercise, the student will be able to: 1) Describe the role of photosynthetic organisms. 2) Discuss the contributions of van Helmont, Priestley, and Ingenhousz on the history of understanding photosynthesis. 3) Compare and contrast the visible and electromagnetic spectrum 4) Discuss the role of pigments in photosynthesis. 5) Discuss the internal anatomy of a leaf 6) Describe the role of the stomata in a leaf 7) Describe the function and organization of chloroplasts. 8) Discuss the location and products of the light – dependent and light – independent reactions. Overview: It is time that plants and other photosynthetic organisms receive a much – deserved “thank you”. Without them we would not exist. On the early Earth, primitive photosynthetic bacteria and algae produced copious amounts of oxygen, changing the atmosphere forever and making it conducive to the development of more complex life forms. It is hard to believe, but the algae that inhabit primarily aquatic environments produce nearly 80% of the oxygen in the atmosphere today and serve as the basis of many food chains. Plants such as grasses, shrubs, and trees dominate terrestrial landscapes, providing habitat, food, and commercial products. Plants are eloquent green machines that harness sunlight and produce carbohydrates and oxygen necessary for the plants themselves, as well as other living things. This remarkable process is termed photosynthesis. Keep in mind that photosynthesis is not the opposite counterpart to respiration. Early philosophers and scientists were intrigued by the mystery of plant growth. Noted Greek scientist such as Aristotle and Theophrastus thought that plants obtained their essential, nutrients exclusively from the soil. Although partially true, it took several centuries for the mystery of plant growth to unfold. The first step in understanding the mystery was taken by a Flemish physician Jan Baptista van Helmont, in the mid – 1600s. he grew a willow tree weighing 2.5 kg in a pot filled with 91 kg of rich soil. Van Helmont provided the tree with water periodically. After 5 years, the tree weighed nearly 75 kg and the soil lost only 57 g of weight. He concluded that the tree gained weight from the water, not the soil. Although he did not understand the role of sunlight and carbon dioxide in plant growth, he initiated the scientific study of plant biology. In the 1770s, the English chemist Joseph Priestley performed a series of unique experiments emphasizing the importance of atmospheric gases in plant growth. Eventually, 1 Priestley was credited with the discovery of oxygen. Building upon the works of Priestley and the “founder of modern chemistry” Antoine Lavoisier, the Dutch physician Jan Ingenhousz hypothesized that plants utilize sunlight to split carbon dioxide and use the carbon for growth. He also concluded that plants expelled oxygen as a waste product. As the result of his work, Ingenhousz is credited with describing photosynthesis. In the mid 1800s, the German botanist Julius von Sachs discovered chloroplasts and their role in photosynthesis. The light reaction, or light – dependent reaction, of photosynthesis was described by Theodor Engelmann in the 1880s. in 1940, Melvin Calvin traced the route of carbon throughout the entire process of photosynthesis. Sunlight powers photosynthesis. Using a prism, the English physicist Sir Isaac Newton demonstrated that white light consists of a variety of color ranging from red at one end of the visible spectrum to violet at the other end. In the mid 1800s, James Clerk Maxwell illustrated that the visible spectrum was a minute portion of a continuous spectrum, or electromagnetic spectrum, that includes radio waves, visible light, x – rays, and cosmic rays. Radiations of the spectrum travel in waves measured in nanometers (1 nm = 10−9m). Radiations with longer wavelengths (radio waves) have less energy, and those with shorter wavelengths (x –rays) have more energy. 1. Observe the image of the electromagnetic spectrum above. What has more energy, a radio wave signal or a gamma ray? ___________________________________________ 2. Observe the image of the electromagnetic spectrum above. What has more energy, green light of purple light? _______________________________________________________ 3. If you go scuba diving, how do the colors shift with depth? Why? ___________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2 For an organism to utilize light energy, it has to be absorbed. In living systems, pigments absorb light energy. Some pigments, such as melanin, absorb all wavelengths of light, and they appear black. At the other end of the spectrum, many pigments absorb only certain wavelengths of light and reflect the other wavelengths. The light absorption spectrum of a pigment illustrates the wavelengths that are absorbed; for example, green leaves contain the pigment chlorophyll which reflects the green portion of the spectrum. Chlorophyll is the most important pigments in photosynthesis. Several types of chlorophyll exist in nature. Chlorophyll a is the main photosynthetic pigment in some cyanobacteria and in plants. Other pigments important in plants but not involved directly in photosynthesis are called accessory pigments. Xanthophyll is a yellowish pigments. (i.e. fall leaves), and carotene is an orange pigment (i.e. carrots). Chlorophyll b is considered an accessory pigment in plants, broadening the spectrum that can be used in photosynthesis. Leaves are the most conspicuous part of a plant. They vary tremendously in shape and size, and some large trees have more than 100,000 leaves. One of the major functions of a leaf is as a photosynthetic factory. The internal anatomy of a typical leaf is complex. A waxy cuticle covers the upper side of the leaf, and an epidermis completes the upper and lower layers of a typical leaf. Scattered primarily throughout the lower epidermis are stomata (sing, stoma) which are tiny openings regulated by guard cells. The stomata allow the carbon dioxide from the atmosphere to enter the leaf. The center of the leaf consists of the mesophyll, composed of palisade parenchyma and spongy parenchyma. The palisade parenchyma is columnar in shape and usually appears beneath the upper epidermis. The spongy parenchyma is loosely packed and surrounded by numerous air spaces. Most of the photosynthetic activity occurs in the cells of the palisade parenchyma. Chloroplasts reside within plant cells and serve as the organelles of photosynthesis. A chloroplast consists of two outer membranes that surround a semifluid matrix called the stroma. A third membrane system forms a series of flattened sacs called thylakoids. In some chloroplasts, the thylakoids become stacked, forming a granum. Pigment molecules embedded in the membrane of the thylakoids initiate photosynthesis. Sugars are synthesized in the stroma. 3 The overall reaction for photosynthesis is: This reaction is the result of a series of chemical reactions that are controlled and carried out by specific enzymes. These reactions of photosynthesis are divided into two distinct metabolic pathways: 1) In the light reaction, or light – dependent reactions, the pigments chlorophyll absorbs light energy from sunlight and produces ATP, the coenzyme NADPH, and oxygen. The light – independent reaction takes place in the thylakoid membrane of the chloroplasts. 2) The dark reaction, also known as the light – independent reaction or Calvin cycle, takes place in the stroma of the chloroplasts. It is responsible for the fixing of a carbohydrate (glucose). 4 Photosynthetic organisms are estimated to produce as much as 200 billion metric tons of carbohydrate yearly. During aerobic respiration, plants and other organisms use the oxygen produced as a byproduct of photosynthesis. The carbohydrates produced during photosynthesis are converted into many plant products such as fiber, wood, and other structural materials. In addition, the simple sugars the plant produces can be converted into disaccharides and polysaccharides, such as starch for energy storage. Sugars also are utilized in the synthesis or amino acids to form proteins and other cellular components. Life on Earth depends on the ability of photosynthetic organisms to convert the radiant energy of the sun into ATP, oxygen, and other nutrients. 5 EXTERNAL STRUCTURE OF A LEAF Photosynthetic reactions usually take place in the leaves. Leaves generally consist of a blade and a petiole. The petiole attaches the fattened blade to the stem. In this activity, you will examine a typical dicot leaf. PHOTOSYNTHESIS Photosynthesis is the process by which light energy converts inorganic compounds to organic substances with the subsequent release of elemental oxygen. It may very well be the most important biological event sustaining life. Without it, most living things would starve, and atmospheric oxygen would become depleted to a level incapable of supporting animal life. Ultimately, the source of light energy is the sun, although on a small scale we can substitute artificial light. Nutritionally, two types of organisms exist in our world, autotrophs and heterotrophs. Autotrophs (auto means self, troph means feeding) synthesize organic molecules (carbohydrates) from inorganic carbon dioxide. The vast majority of autotrophs are the photosynthetic organisms that you are familiar with – plants, as well as some protistans and bacteria. There organisms use light energy to produce carbohydrates. (A few bacterial produce their organic carbon compounds chemosynthetically, that is, using chemical energy) By contrast, heterotrophs must rely directly or indirectly on autotrophs for, their nutritional carbon and metabolic energy. Heterotrophs include animals, fungi, many protistans, and most bacteria. In both autotrophs and heterotrophs, carbohydrates originally produced by photosynthesis are broken down by cellular respiration, releasing the energy captured from the sun for metabolic needs. 6 The photosynthetic reaction is often conveniently summarized by the equation: 6CO2 + 12H2O (+ light energy, enzymes and pigments) C6H12O6 + 6H2O + 6O2 Although glucose is often produced during photosynthesis, it is usually converted to another transport or storages compound unless it is used immediately for carbohydrates metabolism. In plants and many protistans, the most common storage carbohydrate is starch, a compound made up of numerous glucose units linked together. Starch is designated by the chemical formula (C6H12O6). Most plants transport carbohydrate as sucrose. The following experiments will give you a better understanding of the principles of photosynthesis. PAPER CHROMATOGRAPHY Plants have a variety of pigments that are necessary for photosynthesis to occur. These pigments, chlorophyll a, chlorophyll b, and accessory pigments such as carotene and xanthophyll, absorb light. The accessory pigments ultimately transmit the light energy they absorb to chlorophyll a. We can easily separate pigments by using a technique called paper chromatography. Pigment is applied as a spot to one end of the paper; that end is put into a solvent (just below the spot). The solvent moves up the paper, taking the pigment particles with it. The properties that affect how far and how fast a particular pigment’s molecules will move up the paper with the solvent are: The Properties of Pigments Absorption How well the pigment molecules stick to or absorb in the paper (the molecules that absorb better tend to move slower) The size of the pigment in the solvent (larger molecules tend to move more slowly Size than smaller ones) Solubility Solubility of the pigment in the solvent (more – soluble molecules move faster and farther) We can identify each pigment by determining its Rf (ratio of fronts) value. The Rf value describes the relationship between the distance moved by the pigment and the distance moved by the solvent, and is calculated as follows: 𝑹𝑓 = 𝐷𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑚𝑜𝑣𝑒 𝑏𝑦 𝑡ℎ𝑒 𝑝𝑖𝑔𝑚𝑒𝑛𝑡 𝐷𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑚𝑜𝑣𝑒 𝑏𝑦 𝑡ℎ𝑒 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 Materials Chromatography jar (containing one inch filled with the 9:1 solution of 10% Acetone in Petroleum Ether) Chromatography paper Hematocrit tube Paintbrush (fine – tip) Color Pencils Forceps Calculator Chloroplast pigment solution Metric Ruler 7 Procedure 1) Obtain a chromatography paper. [Warning: Use glove for this experiment because the oil from your hands may interfere with the development of the chromatogram results.] 2) Using a metric ruler, draw a pencil line (DO NOT USE PEN) measuring 2cm starting from the bottom but leave 0.5 cm away from each side of the chromatography paper as demonstrated on the diagram shown below. 3) Next, draw another pencil line (once again DO NOT USE PEN) measuring 1cm beginning from the top of the chromatography paper as demonstrated on the chromatogram shown below. 4) Using a small paint brush (fine tip) or a hematocrit tube paint a line of chloroplast pigment solution across the paper chromatography on the 2 cm drawn line and let it dry. Repeat this step 10 times to obtain a better result. [NOTE: before applying a second application of pigment solution, let pigment dry before applying another application of the chloroplast pigment solution.] 8 5) Place your chromatography paper into a chromatography jar (containing one inch filled with the 9:1 solution of the 10% Acetone in Petroleum Ether) with the pigment at the bottom and close the jar (see figures below). [Caution: the solvent and the pigment solution are highly flammable. Keep them away from open flames.] Accessory pigments Accessory pigments 6) Once the solvent reaches the 1 cm pencil marking, remove the paper chromatography with forceps and place it on a paper towel. 7) Calculate the 𝑹𝒇 value for each pigment by measuring the distance of the solvent from its origin to the highest point it traveled on the paper. Then measure the distance the different pigments travel from the origin (2 cm pencil mark) to the center of each pigment band. Calculate the 𝑹𝒇 value by using the formula below. Record your measurement on table 10.1 9 Band # 1 2 3 4 Pigment Carotene Xantophyll Chlorophyll a Chlorophyll b 5 Solvent (9:1) Petroleum Ether in Acetone Color Orange – Yellow Yellow Blue – green Olive to yellowish-green Clear Migration Distance (mm) 𝑹𝒇 Value 1. What does a small 𝑅𝑓 value tell you about the characteristic of the moving molecules? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2. Which is more soluble in the chromatography solvent, xanthophylls or chlorophyll a? ______________________________________________________________________________ 3. Why did the plant extract appear green if yellow xanthophylls were present? ______________________________________________________________________________ ______________________________________________________________________________ 4. Based on your observations during this experiment, what is responsible for the change in leaf color during the fall? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ CARBON DIOXIDE UPTAKE DURING PHOTOSYNTHESIS Recall that plants require carbon dioxide to produce carbohydrates (sugar0 during the light independent reaction of the photosynthesis. To detect the uptake of carbon dioxide by the plant, you will use a pH indicator solution. An indicator is a molecule that changes color depending on pH. In this experiment you will be using a phenol red solution. Phenol red turns yellow when adding carbon dioxide to its substance making it an acidic solution (pH < 7.0) and when red there is no presence of carbon dioxide making it a neutral to basic solution (pH > 7.0) In order to see this reaction take place, your breath will provide the carbon dioxide to a solution of phenol red. This results in the following chemical reaction. 𝐇𝟐 𝐎 + 𝐂𝐎𝟐 𝐇𝟐 𝐂𝐎𝟑 𝐇 + + 𝐇𝐂𝐎𝟑 10 A plant added to the solution can “fix” the carbon dioxide (that you just exhaled into) and this will cause the pH to rise (become more alkaline). Your solution then turns red. Materials 2 Large test Tubes Test tube Rack Lamp 400 mL Beaker Straw 10 cm of Elodea Procedure 1) Professor will provide the students with the Phenol Red Solution in the 400 mL Beaker 2) Fill one test tube # 1 with Phenol red solution 3) With the straw, blow on the remaining phenol red until it turns yellow, then add the yellow phenol red to test tube #2. 4) Insert a 10 cm Elodea on test tube #2. 5) Place both test tubes in front of a bright light for 60 – 90 minutes. [Note: This will ensure that the plant has enough supplies of ATP and NADPH necessary for the light – independent reaction.] Test Tube 1 Phenol red (-) control. Test Tube 2 Phenol red that turned yellow (Co2) + Elodea. This is your experimental control. 1. Has the color in either test tube changed? If so why? Explain. ______________________________________________________________________________ ______________________________________________________________________________ 2. Explain. What is Carbon fixation? ______________________________________________________________________________ ______________________________________________________________________________ 3. Did you see a change in color of the phenol red test tube with no elodea added? Why or why not? _____________________________________________________________________________________ _____________________________________________________________________________________ 11 TEST FOR STARCH As indicated by the overall formula of photosynthesis, one end product is a carbohydrate (𝐂𝐇2 𝐎). But a number of different carbohydrates have the empirical formula𝐂𝐇2 𝐎. In this experiment, you will perform a simple test to visually distinguish between two different carbohydrates and water. Materials Bottle of Lugol’s solution Bottle of double distilled water Bottle of Starch solution Bottle of Glucose solution Three depression (spot) plates Procedure 1) Place one drop of double distilled water, starch and glucose in the three depression (spot) plate. Add one drop of Lugol’s solution in each spot. 2) Record your result for the presence of starch on the following table. Solution [(+/-) for the presence of starch] Double distilled water Starch Glucose RELATIONSHIP BETWEEN LIGHT AND PHOTOSYNTHESIS PRODUCTS This experiment addresses the hypothesis that light is necessary for photosynthesis to proceed. Materials Per group: Sharpie 1000 mL Beaker (fill with distilled water and glass beads) Hot plate Long forceps 2 petri Dishes Lugol’s Solution Per Lab: Hot Plate 1000 mL Beaker (fill with ethanol and glass beads cover with foil paper) Light – grown geranium plant with leaves cover or marks Dark – grown geranium plant with leaves cover or masks 12 Procedures 1) Observe the two geranium plants available. One plant has been growing on bright light for several hours. The other has been kept in the dark for a day or more. Bath leaves have an area cover with a piece of foil paper. 2) Write a prediction regarding the presence of starch and the activity of photosynthesis for each growing condition leaf in the following table. Geranium Plant Growing Condition Light – Growing Plant Dark – Growing Plant Covered Area Uncovered Area 3) Select a leaf from one of the two geranium plants. Pigment present in the pants must be removed before a test for starch can be performed. Kill the leaf and extract the pigment. a) Turn on the hot plate and set it to high setting. Allow the water to come to a boil. b) With a sharpie, label one Petri dish light – grown plant and the other dark – grown plant c) Remove a leaf from each condition plant and take it to your station. d) Remove the piece of foul paper from both leaves. e) Place one leaf in the beaker of boiling water for about a minute. This kills the tissue and breaks down internal membranes (cell wall, plasma membrane, and vacuolar membrane) f) Remove the wilted leaf from the water with the long forceps and place it on the Petri dish. g) Place the wilted leaf in the beaker of boiling alcohol and cover it. Let it set for about a minute. This will extract the photosynthetic pigments from the plant tissues. When the pigments have been extracted, the liquid will appear green, and the leaf will appear to be mostly bleached. h) Remove the leaf from the alcohol with forceps, and dip it back into the boiling water for about 15 seconds to soften and to remove excess alcohol. 4) Test the plant for the presence of starch: a) Place killed leaf in a Petri dish and pour 2 mL of Iodine (Lugol’s) solution on top of the leaf. b) Let it soak in the Iodine solution for a couple of minutes. Rinse the leaf and Petri dish with water to remove the iodine solution in order to observe the pattern of staining. c) Record your results in the given table d) Repeat steps 3e – 4c for the other leaf. Geranium Plant Growing Condition Light – Growing Plant Dark – Growing Plant Covered Area Uncovered Area 1. What does the blue – black coloration of the leaf show? ______________________________________________________________________________ ______________________________________________________________________________ 2. Why did the masked area fail to stain? ______________________________________________________________________________ ______________________________________________________________________________ 3. Write the conclusion accepting or rejecting the hypothesis. ______________________________________________________________________________ 13 ABSORPTION OF LIGHT BY CHLOROPLAST EXTRACT People tend to think of sunlight as being white. However, as you will see in this experiment, white light consists of continuum of wavelengths. If we see light of just one wavelength, the light will appear colored. When light hits a pigmented surface, some of the wavelengths are absorbed and others are reflected or transmitted. In this experiment, you will discover which wavelength are absorbed, transmitted, or reflected by particular pigments; among them the photosynthetic pigment chlorophyll. Materials Spectroscope Colored filters (Blue, green and red) Colored pencils Small test tube containing pigment extract Procedure One way to separate light into its component parts is to view the light through a spectroscope. The spectroscope contains a prism that causes a spectrum of color to form. A nanometer scale is imposed on the spectrum to indicate the wavelength of each component of white light. A) Observe the spectrum of white light given off by a light bulb through the spectroscope. With the colors violet, blue, green, yellow, orange, and red record what you see on the given table. B) Observe the spectrum produced by the three colored filters using the spectroscope. 1. Which color or colors are absorbed when the red filter is placed viewed through the spectroscope. ______________________________________________________________________________ ______________________________________________________________________________ 2. Which color or colors are absorbed when the blue filter is placed viewed through the spectroscope. ______________________________________________________________________________ ______________________________________________________________________________ 14 3. Which color or colors are absorbed when the green filter is placed viewed through the spectroscope. ______________________________________________________________________________ ______________________________________________________________________________ 4. Make a general statement concerning the color of a pigment (filter) and absorption of light by that pigment. ______________________________________________________________________________ ______________________________________________________________________________ C) Now obtain a small test tube containing spinach chloroplast pigment extract and place it between the light source the spectroscope. By adjusting the height of the tube so that the upper portion of the light passes through the pigment extract and the lower portion is white light, you can compare the absorption spectrum of the pigment extract with the spectrum of white light. An absorption spectrum is a spectrum of light waves absorbed by a particular pigment. By contrast, the wavelengths that pass through the pigment extract and are visible in the spectroscope make up the transmission spectrum of the pigment (Note: see picture below and picture on page 14). D) Using the colored pencils, record the transmission spectrum of the chloroplast extract on the following table. 1. How does the absorption spectrum of the chloroplast extract compare with the absorption spectrum of the green filter? ______________________________________________________________________________ 2. How might you explain the difference in absorption by the green filter and by the chloroplast pigment extract? [Hint: see electromagnetic spectrum on page 14 and paper chromatography table on page 10.] ______________________________________________________________________________ ______________________________________________________________________________ 15 ___________________________________________ Last Name, First Name [lab partner N0. 1] ____________________________________________ Last Name, First Name [lab partner N0. 2] _______________________________ _______________________________ Last Name, First Name [lab partner N0. 3] ___________________________ Last Name, First Name [lab ______________________ Section partner N0. 4] ______________________ group # Date REVIEW QUESTIONS 1. Write the equation for photosynthesis _____________________________________________________________________________ 2. Why photosynthesis is considered the most important chemical reaction of Earth? _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ 3. Compare and contrast the visible and electromagnetic spectrum. _____________________________________________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ 4. Name and describe three pigments found in the labs in this chapter. (1) _______________________________________________________________________ _______________________________________________________________________ (2) _______________________________________________________________________ _______________________________________________________________________ (3) _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ 16 5. Sketch and label the basic internal anatomy of a leaf. 1. 2. 3. 4. 5. Describe the role of the stomata in leaves _____________________________________________________________________________ _____________________________________________________________________________ 6. Sketch and label a chloroplast, including the membranes, stroma, thylakoids, central vacuole, and nucleus. 6. grana. _____________________________________________________________________________ _____________________________________________________________________________ 17 7. Describe the light – dependent and light – independent reactions of photosynthesis? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 8. How is chromatography used to study plant pigments? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 9. Discuss the succession of C3 and C4 plants in your yard or an open field through the four seasons. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 10. Describe several products of photosynthesis that are important to humans. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 18