6th grade MSP Wiki Integrated Lesson
advertisement

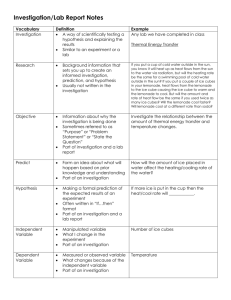
6th grade MSP Lesson on Heat Convection and Mathematical Expressions Math Standards 6.EE.A.2 - Write, read, and evaluate expressions in which letters stand for numbers. 6.EE.A.3 Apply the properties of operations to generate equivalent expressions. 6.EE.A.4 Identify when two expressions are equivalent. Science Standards SPI 0607.8.3 Describe how temperature differences in the ocean account for currents. SPI 0607.8.1 Analyze data to identify events associated with heat convection in the atmosphere. SPI 0607.8.2 Recognize the connection between the sun’s energy and the wind. Science Content Knowledge Heat is energy in transit from warmer systems to colder systems. The energy always moves from a warmer system to a colder system. The energy which is moving from one system to another is known as heat. The transfer or dispersion of heat can occur by means of three main mechanisms, conduction, convection and radiation CONVECTION: Flow of heat through currents within a fluid (liquid or gas). Convection is the displacement of volumes of a substance in a liquid or gaseous phase. When a mass of a fluid is heated up, for example when it is in contact with a warmer surface, its molecules are carried away and scattered causing that the mass of that fluid becomes less dense. For this reason, the warmed mass will be displaced vertically and/or horizontally, while the colder and denser mass of fluid goes down (the low-kinetic-energy molecules displace the molecules in high-kineticenergy states). Through this process, the molecules of the hot fluid transfer heat continuously toward the volumes of the colder fluid. For example, when heating up water on a stove, the volume of water at the bottom of the pot will be warmed up by conduction from the metallic bottom of the pot and its density decreases. Given that it gets lesser dense, it shifts upwards up to the surface of the volume of water and displaces the upper -colder and denser- mass of water downwards, to the bottom of the pot. Source http://www.biocab.org/Heat_Transfer.html Three Video Clips: 1. Temperature vs. Heat http://www.neok12.com/video/Heat-Temperature/zX585a036141017f07436451.htm Bucket of water at 50 degrees Celsius vs. a cup of water at 100 degrees Celsius. Place these into a cool pool and check if it warms the water up. 2. Temperature and Heat: Discussion http://www.neok12.com/video/Heat-Temperature/zX615658021a7c56507d7977.htm Observe 3 beakers with the same temperature but different amount of water. Temperature is based on the speed of the particles moving. Temperature measures the kinetic energy of a substance. Heat is determined by temperature and amount of substance. 3. Demonstrations of Heat Transfer by Conduction, Convection and Radiation http://www.neok12.com/video/Heat-Temperature/zX5b790457005d520e055f02.htm Another Resource for Science Standards: Sun’s energy, Air Moving, Types of Wind, Currents http://www.projectsharetexas.org/resource/sun-and-convection-currents-ontrack-sciencegrade-8-module-3-lesson-10 Integrated Activity Private Think Time: What does happen when water is heated in the microwave? What does happen when ice is added to water? Share out predictions. Teacher asks: How can you investigate this process? How can you test your hypothesis? Part I of the Investigation: 1. Measure 200 ml of water and place in microwave safe container. Measure its initial room temperature. Heat the water in the microwave for 1 minute. Record temperature after heating time. 2. Repeat same process as step no. 1; but double the heating time. 3. Repeat same process as step no. 1; but increase the heating time by adding 2 more minutes. 4. Repeat same process as step no. 1; but increase the heating time by adding 3 additional minutes. 5. Complete table below and calculate the change in temperature by subtracting the initial temperature from the final temperature. What do these procedural phrases mean in mathematical terms? Write the expressions: Direction Expression double the heating time increase the heating time by adding 2 more minutes increase the heating time by adding 3 additional minutes Subtracting the initial temperature from the final temperature Ask students, "What action is happening in this phrase?" (Answer: We are decreasing, going down, subtracting). Then ask, "What operation indicates this action?" (Answer: Subtraction) Conduct Investigation and collect data: Trial Water Heating Initial Final Change in Amount Time Temperature Temperature Temperature 1 2 3 4 200 ml 200 ml 200 ml 200 ml 1 min 2 min 3 min 4 min What is the constant(s) in this investigation? What is the variable(s) in this investigation? Analyze data collected. What will happen after warming the water in the microwave for 7 min? Use evidence from data collected to support your answer. Part II of the Investigation: 1. Measure 200 ml of water and place in container. Record its initial room temperature. Add 5 ice cubes and wait 2 minutes. Record temperature. 2. Repeat same process as step no. 1; but increase the number of ice cubes by the product of the original number and 2. 3. Repeat same process as step no. 1; but increase the number of ice cubes by having 3 times the original amount. 4. Repeat same process as step no. 1; but increase the number of ice cubes by adding 3 times more to the original amount. 5. Complete table below. The final temperature minus the initial temperature will give you the temperature change. What do these procedural phrases mean in mathematical terms? Write the expressions: Direction product of the original number and 2 3 times the original amount adding 3 times more to the original amount final temperature minus the initial temperature Expression Ask students, "What action is happening in this phrase?" (Answer: We are decreasing, going down, subtracting). Then ask, "What operation indicates this action?" (Answer: Subtraction) Conduct Investigation and collect data: Trial Water Number of Initial Final Change in Amount Ice Cubes Temperature Temperature Temperature 1 2 3 4 200 ml 200 ml 200 ml 200 ml 5 10 15 20 What is the constant(s) in this investigation? What is the variable(s) in this investigation? Analyze data collected. What will happen after adding 35 ice cubes to the water? Use evidence from data collected to support your answer. Part III of the Investigation: What will happen when very hot water is placed near very cold water? What will happen to the air surrounding both water containers? 1. Warm up 200 ml of water in a microwave for 4 minutes. Add heated water to a container with 200 ml of room temperature water. 2. Place 35 ice cubes to a container with 200 ml of room temperature water. 3. Locate both containers side by side. 4. Lit an incense stick and place between the two containers. Observe and discuss your findings. Adapted from: http://boyslife.org/hobbies-projects/funstuff/2859/weather-experiments/ 6th grade MSP Sep 2015- CMCSS Training