Supplementary Material (A) (B) (C) Figure S1. Perfusate
Supplementary Material
(A)
100
90
80
70 LCFA colloids
LCFA colloids + amiloride
60
0 30 40 50 60 70
Time (min)
(C) (B)
100 100
90
80
90
80
70 LCFA colloids
LCFA colloids + amiloride
70 LCFA colloids
LCFA colloids + amiloride
60 60
0 30 40 50 60 70 0 30 40 50 60 70
Time (min) Time (min)
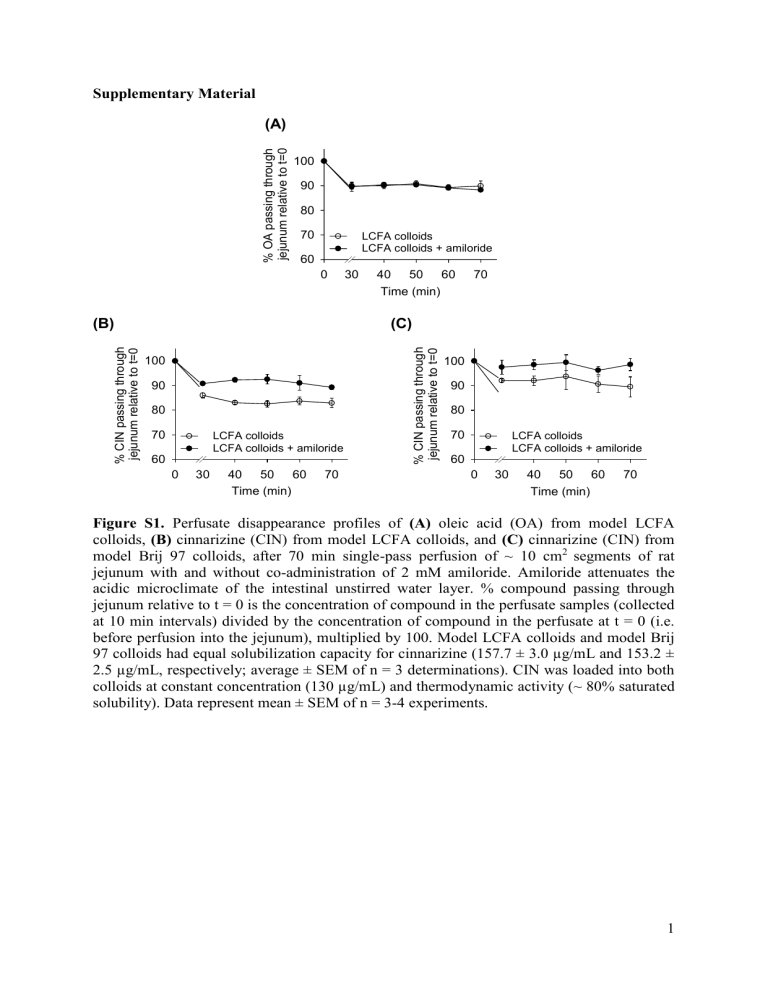
Figure S1. Perfusate disappearance profiles of (A) oleic acid (OA) from model LCFA colloids, (B) cinnarizine (CIN) from model LCFA colloids, and (C) cinnarizine (CIN) from model Brij 97 colloids, after 70 min single-pass perfusion of ~ 10 cm
2 segments of rat jejunum with and without co-administration of 2 mM amiloride. Amiloride attenuates the acidic microclimate of the intestinal unstirred water layer. % compound passing through jejunum relative to t = 0 is the concentration of compound in the perfusate samples (collected at 10 min intervals) divided by the concentration of compound in the perfusate at t = 0 (i.e. before perfusion into the jejunum), multiplied by 100. Model LCFA colloids and model Brij
97 colloids had equal solubilization capacity for cinnarizine (157.7 ± 3.0 µg/mL and 153.2 ±
2.5 µg/mL, respectively; average ± SEM of n = 3 determinations). CIN was loaded into both colloids at constant concentration (130 µg/mL) and thermodynamic activity (~ 80% saturated solubility). Data represent mean ± SEM of n = 3-4 experiments.
1
(A)
No turbidity
(B)
Increasing turbidity *
0% OA
0% MO
(C)
Increasing turbidity *
0.025% w/v OA
0.016% w/v MO
(D)
Increasing turbidity
* *
0.050% w/v OA
0.032% w/v MO
(E)
No turbidity
0.10% w/v OA
0.06% w/v MO
3.09% w/v Brij 97
Figure S2 . The appearance of LCFA-containing intestinal colloids (panel A-D) and 3.09% w/v Brij 97 colloids (panel E) 120 h into cinnarizine equilibrium solubility determination studies, after vials were centrifuged at 2,200 x g for 10 min at 37 °C. LCFA-containing colloids comprised oleic acid (OA) and monoolein (MO) (concentrations as labeled) solubilized in simulated endogenous intestinal fluid (SEIF) containing 4 mM total bile salt, 1 mM lysophosphatidylcholine and 0.25 mM cholesterol. In each panel, the system pH of colloids was, from left to right, 6.30, 5.80, 5.30 and 4.80, respectively. In LCFA-containing systems, the turbidity of samples was observed to increase when system pH was decreased, up to a point where colloids were destabilized and underwent phase separation into an
2
aqueous phase and an undispersed oil phase (undispersed oil phase floats on top of aqueous phase and may not be seen clearly in pictures), at which point the aqueous phase appeared clear again. * denotes colloidal systems where phase separation into an aqueous phase and undispersed oil phase was evident. Since the concentration of bile components was held constant, phase separation of colloidal system with 0.10% w/v OA (Panel D) occurred at a higher pH than systems containing 0.05 and 0.025% w/v OA (Panel B and C). Turbidity did not develop in SEIF containing 0% OA (Panel A) or 3.09% w/v Brij 97 colloids (Panel E).
The white mass at the bottom of vials is the excess solid cinnarizine added during the solubility determinations. The pictures illustrate the impact of the acidic microclimate on the microstructure of LCFA-containing intestinal colloids, where exposure of LCFA colloids to the microclimate pH leads to protonation of LCFA, increasing the ratio of unionized
LCFA:ionized LCFA and resulting in the formation of larger colloids with reduced thermodynamic stability in vitro . In vivo , the increase in LCFA protonation and LCFA thermodynamic activity is expected to lead to enhanced micellar dissociation and absorption.
3
(A)
100
90
80
70
60
50
0 30 40 50 60 70
Time (min)
(B)
100
90
80
70
60
50
0 30 40 50 60 70
Time (min)
Figure S3. Perfusate disappearance (% drug dose passing through jejunum) profiles of (A) oleic acid (OA) and (B) cinnarizine (CIN) when model LCFA colloids were perfused via single-pass through an isolated rat jejunal segment (~ 10 cm 2 ), with and without 1:1 v/v coperfusion with donor rat bile, in the absence and presence of 2 mM amiloride. * Data in the
absence of amiloride are reproduced from (1). % compound passing through jejunum relative
to t = 0 is the concentration of compound in the perfusate samples (collected at 10 min intervals) divided by the concentration of compound in the perfusate at t = 0 (i.e. before perfusion into the jejunum), multiplied by 100. Co-perfusion of model LCFA colloids with rat bile generates drug supersaturation in situ within the perfused jejunal segment. The degree of drug precipitation within the perfusate is represented by the difference in CIN perfusate concentration pre- and post-centrifugation.
SS represent mean ± SEM of n = 3-4 rats. denotes drug supersaturation in perfusate. Data
4
(A) (B)
Model LCFA colloids Model Brij 97 colloids
Figure S4 . Appearance of cinnarizine-loaded model LCFA colloids (Panel A) and model
Brij 97 colloids (Panel B) immediately after the addition of one drop of 20% v/v H
3
PO
4
.
Turbidity instantly developed in model LCFA colloids while model Brij 97 colloids remained optically clear. The addition of H
3
PO
4
reduced the system pH of model LCFA colloids and
Brij 97 colloids from 6.30 to 5.92, and 6.30 to 6.01, respectively. Magnetic stirrers are present at the bottom of vials. This figure visually depicts the effect of protonation of oleic acid (OA) when model LCFA colloids are exposed to acidic conditions. An increase in the ratio of unionized OA:ionized OA is thought to enhance the thermodynamic potential of OA, and induce a micelle to emulsion transition in the colloids.
5
REFERENCE
1. Yeap YY, Trevaskis NL, Porter CJH. The potential for drug supersaturation during intestinal processing of lipid-based formulations may be enhanced for basic drugs. Molecular
Pharm. 2013 (in press) DOI: 10.1021/mp400035z.
6