Temperature_Change_Lab_
advertisement

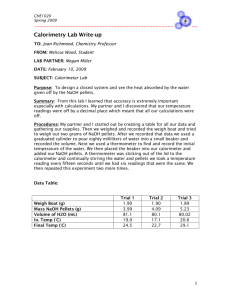
Temperature Change/Calorimetry Lab Chemistry Challenge: Can you determine if the temperature increases or decreases in a chemical reaction? Can you calculate the amount of heat released/gained by chemical compound or reaction? Materials: Goggles Citric Acid Sodium bicarbonate Calcium chloride Water—tap water is fine Graduated cylinder Thermometer 2 small spoons 2 small Styrofoam cups Procedure: 1. Assemble the calorimeter as follows: a. Take a Styrofoam cup and insert another Styrofoam inside the cup. b. Cover the calorimeter with a cardboard lid and place the thermometer inside the hole in the lid. c. Obtain the mass of the empty cylinder. Part I: 2. Using a graduated cylinder, measure 20ml of water and place it into the calorimeter. 3. Record the mass of the calorimeter and the water solution. 4. Place 2 small spoonful of citric acid into the Styrofoam cup. Stir the solution until the citric acid dissolves. 5. Place a thermometer in the “citric acid” solution and record the temperature as the “initial temperature.” Keep the thermometer in the solution. 6. Add 1 spoonful of sodium bicarbonate to the solution and stir the solution. Record the highest or lowest temperature as the “final temperature.” 7. Place the solutions down the drain with plenty of water. Solid waste can be disposed of in the trashcan. Be sure to completely dry the calorimeter. Part II: 8. Measure 20 ml of water using a graduated cylinder and place the water into the calorimeter. Obtain the mass of the empty calorimeter 9. Record the mass of the calorimeter plus the water solution. 10. Place 2 small spoonfuls of sodium bicarbonate into the cup and stir until the compound has completely dissolved. 11. Place a thermometer in the “sodium carbonate” solution (be sure the thermometer is clean and dry) and record the temperature as the “initial temperature.” Leave the thermometer in the solution. 12. Place 1 spoonful of calcium chloride into the solution and stir. Record the highest/lowest temperature reached as the “final temperature.” 13. Place the solutions down the drain with plenty of water. Solid waste can be disposed of in the trashcan. Be sure to completely dry the calorimeter. Part III: 14. Measure 20ml of water using a graduated cylinder and place the water in the calorimeter. Obtain the mass of the empty calorimeter. 11. Record the initial temperature of the water using your thermometer. 12. Place 2 small spoonfuls of calcium chloride into the calorimeter and stir. Record the highest or lowest temperature reached as the “final temperature” in the fourth column. 15. Clean your lab area and WASH YOUR HANDS! Chemical Reaction Citric acid + sodium bicarbonate Sodium bicarbonate + calcium chloride Calcium chloride + Water Mass of empty calorimeter Mass of calorimeter + water solution Starting Temperature (C) Final Temperature (C) Difference in Temperature Did the temperature go up or down? Is the chemical reaction exothermic or endothermic? Questions: 1. List your observations. 2. Calculate the enthalpy of the reaction between calcium chloride and water. (q= smT, use the specific heat of water and the mass of the water solution)