Enthalpy and Calorimetry
advertisement

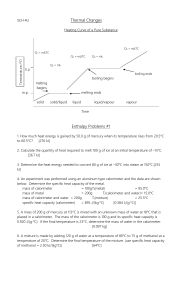
Enthalpy and Calorimetry Chapter 5 part 2 Enthalpy • H is heat under constant pressure or • H=qP • H=E+PV • And therefore • ΔH= ΔE+P ΔV • ΔH=Hfinal-Hinitial Example: • When 1 mole of methane is burned at a constant pressure. 890kJ of energy is released as heat. Calculate the ΔH for a process in which a 5.8g sample of methane is burned at constant pressure. Answer • ΔH= -890kJ/mol • CH4 is methane, MM = 16 g/mol • 5.8g/16g mol-1 = 0.36 mol • 0.36 mol CH4 x -890kJ/mol CH4 = -320kJ Calorimetry • The science of measuring heat. • The device used to measure heat changes associated with chemical reaction is a calorimeter Heat Capacity • Specific heat capacity • Heat capacity of an is the heat capacity object is the heat per gram of a absorbed by the change substance or in temperature or • Csp=C/gram • C=(heat absorbed)/(ΔT) • Molar heat capacity is the heat capacity per mole of a substance or • Cmol=C/mole Calorimeters • Constant pressure calorimeter • Calculations (per gram) • Csp* mass*ΔT= ΔH Remember: • the specific heat and the mass are of the same object. • The measurement is of the surrounding water, not the actual system so the sign is reversed. Example: • When 1.00 L of 1.00M Ba(NO3)2 solution at 25.0 °C is mixed with 1.00 L of 1.00 M Na2SO4 solution at 25 °C in a calorimeter, the white solid BaSO4 forms and the temperature of the mixture increases to 28.1 °C . Assuming that the calorimeter absorbs only a negligible amount of heat and the specific heat of the solution is 4.18J/°Cg and the density of the solution is 1.00g/mL, calculate the enthalpy change per mole of BaSO4 formed. • Ba2+ (aq) + SO4 2- (aq)→BaSO4 (s) • Mass of solution =2 liters = 2000 grams • Csp= 4.18J/°Cg • ΔT=28.1-25.0 =3.1 °C • qsurroundings = 2.6 x104J • qsystem= -2.6 x104J Bomb Calorimeter • This is also known as a constant volume calorimeter. • Since it is under constant volume, but there is a change in temperature, the pressure is not constant. Bomb Calorimeter • Note: Since there is no change in volume, then no work is done. • The constant volume calorimeter measures the system directly. • The specific heat is of the calorimeter itself. Example • In comparing potential fuels a bomb calorimeter with a specific heat of 11.3kJ/ °C was employed. When a 1.50 g sample of methane was burned with an excess of oxygen in the calorimeter, the temperature increased by 7.3 °C . When a 1.15 g sample of hydrogen gas was burned with an excess of oxygen, the temperature increase was 14.3 °C. Calculate the energy of combustion per gram for these two fuels. • Methane: released energy from 1.5 gram. • =(11.3 kJ/g °C)(7.3 °C) • =83kJ • Per gram • 83kj/1.5g=55kJ/g • Hydrogen: released energy from 1.15 g. • =(11.3 kJ/g °C)(14.3 °C) • =162 kJ • Per gram • 162kJ/1.15 g= 141 kJ/g Hess’s Law • Since enthalpy is a state function, then • In going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or a series of steps. Hess’s Law