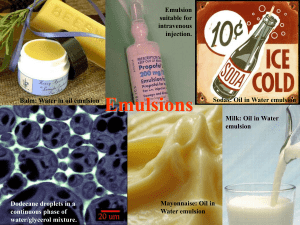
Chapter 2: Emulsions - Performance Chemical Company
advertisement

Chapter 2: Emulsions Chapter 2: Emulsions 2 .1 Pr oble m The most important objective of any oil production facility is the separation of water and other foreign materials from the produced crude. The breaking of these “crude oil and water emulsions” constitutes one of the more challenging problems in today’s oil producing industry. During the productive life of an oil or gas well, a stage is reached when water will be coproduced in unacceptable quantities. This water coexists with the hydrocarbons in the reservoir and gradually infiltrates into the hydrocarbon-bearing region of the formation. Eventually water becomes part of the production from the wells regardless of the method of recovery. Figure 1 on the next page shows a simplified view of how water may be produced. In the early life of the producing field some wells that are drilled close to the oil-water contact level will begin to produce water. Other wells drilled higher in the reservoir will produce dry oil. Later, as the oil in the reservoir becomes depleted and the water expands upward, the oil-water interface level rises until the wells higher in the reservoir begin to produce water. In some cases, it is possible to exclude some or most of the water by plugging back the lower part of the wellbore with cement and perforating an interval higher up in the formation. This can at least delay water encroachment for a time. Secondary or tertiary recovery methods are another cause of water encroachment. These recovery methods are employed to increase the amount of oil recovered from the reservoir, and they involve many different techniques. A number of these methods require the injection of water or steam into the reservoir, and of course, the water is often produced again with the oil. 2-2 Chapter 2: Emulsions Figure 1 Producing Wells Oil Oil - Water Contact W at e r Oil leaving the producing facility has to meet a low water content specification. Too high a level of produced water in the exported oil would severely reduce pumping and other transport capacity. Even a small percentage of emulsified water in crude oil increases the cost of pumping due to the larger volume and the higher viscosity of the oil. In addition, the high salinity of the water would cause corrosion and scaling in downstream operations. It is therefore necessary to remove the water and associated salts from the crude oil. Production of immiscible oil and water through wellhead chokes and valves, along with the simultaneous action of shear and pressure reduction, often produces stable water-in-oil mixtures. The relative stability of these mixtures depends upon many factors such as water cut, the nature of salts present, the viscosity of the oil, and in particular, the indigenous surfactants present in the oil. Some of the water does not mix with the oil to give a stable mixture. This “free water” readily separates from the oil. More often, the conditions of production are such that a stable mixture is formed. Such a mixture is called an emulsion and must be specially treated before separation can occur. To appreciate the difficulties associated with the production and treatment of emulsions it is helpful to have some basic knowledge of emulsion theory. 2-3 Chapter 2: Emulsions 2.2 Emulsion Theory An emulsion is a mixture of two immiscible liquids, one of which is dispersed as droplets in the other. The liquid in an emulsion that is broken into droplets is known as the dispersed or internal phase, whereas the liquid surrounding the droplets is called the continuous or external phase. Emulsions formed in oil producing operations are predominantly water-in-oil. 2.2.1 Types of Emulsion Emulsions are classified according to which phase is dispersed and which phase is continuous. 1. Water-in-Oil Emulsions (W/O) Water is dispersed in oil: water is the dispersed or internal phase, and oil is the continuous or external phase. This type is often referred to as a “regular emulsion” or an oil continuous emulsion. Waterin-oil emulsions are the type most frequently encountered when oil and water are produced. An oil-in-water emulsion may contain anywhere from a trace to 90 plus percent water. Treating this type of emulsion is called dehydration. 2. Oil-in-Water Emulsion (O/W) Oil is dispersed in water: oil is the dispersed or internal phase, water is the continuous or external phase. This type can also be called a “reverse emulsion” or water continuous emulsion. These emulsions exist naturally in certain parts of the world. Oil-in-water emulsions can also be encountered in the water that has been separated from the oil during dehydration. Treatment of this type of emulsion is sometimes referred to as de-oiling. 3. Multiphase Emulsions It is common to find both oil-in-water and water-in-oil emulsions occurring simultaneously. This is frequently encountered in slop oil systems and storage tanks where various emulsions have mixed and been allowed to stand for a period of time. It can also result from various secondary and tertiary recovery processes. 2-4 Chapter 2: Emulsions 2.2.2 Formation of Emulsions A stable emulsion is one that will not break down without some form of treating. Three conditions are necessary for the formation of a stable emulsion: 1. The liquids must be immiscible. 2. There must be an emulsifying agent, or emulsifier, present. 3. There must be sufficient agitation to disperse one liquid as droplets in the other. Many emulsions are prepared for commercial use, such as insecticides and medicines. These are made up of two or more liquids that will not normally mix, plus the emulsifying agent. A common household emulsion is mayonnaise. Mayonnaise is made of vegetable oil and vinegar with eggs used as the emulsifying agent. This combination would not remain mixed if the eggs, or some other emulsifying agent, were not present. They could be mixed by violent agitation, but they would soon separate after agitation was stopped. Similarly, to form a stable emulsion of crude and water, an emulsifying agent must be present. The stability of petroleum emulsions depends upon the presence of an emulsifying agent that is soluble, dispersible, or wettable in or by the oil or the water. If the emulsifying agent is soluble, dispersible, or wettable more easily in or by oil than water, then the oil will be the external phase and water the dispersed phase. Whereas, if the emulsifier is soluble, dispersible, or wettable more readily in or by water than oil, then the opposite type of emulsion will be formed. The most common emulsifying agent found in petroleum emulsions include asphaltenes, solid paraffins, resinous substances, napthenic and other oil soluble organic acids, and finely divided materials that are more soluble, wettable, or dispersible in oil than water. Also found are zinc, iron, aluminum sulfates, calcium carbonate, silica, and iron sulfide. These substances are usually found at the interface between the oil and droplets of water in the form of a film around the droplet. Other emulsifying agents may be drilling, stimulation, or production chemicals. These emulsions are referred to as “chemically stabilized emulsions.” Care should be taken in the selection of chemicals to prevent formation of chemically induced emulsions. For example, corrosion inhibitors should be tested for emulsion tendency before a product is selected in order to prevent emulsification of the well during batch treatment. In addition, demulsifiers should be tested for overtreatment during Bottle Testing to prevent the application of a demulsifier which may overtreat or “burn” the oil. The agitation necessary to form most petroleum emulsions is caused by gas bubbling through the oil and water or by the two liquids being forced through relatively small openings, such as chokes, at high velocity. It is a recognized fact that emulsions are formed rarely, if ever, in the oil reservoir, although some may be formed where the water and oil enter the well. 2-5 – Chapter 2: Emulsions Emulsification generally occurs at some stages in production. Various sources of agitation sufficient to cause emulsification may be present between the time when the oil enters the well and the time when they are separated at the surface. There is evidence that emulsions are formed in wells and in the mechanical equipment used in production, or even later in the flowlines on the surface. Undoubtedly, certain methods of production contribute to the formation of emulsions. Naturally, flowing wells produced through chokes and wells produced by gas lift or air lift usually cause the most difficult emulsion problems. Most emulsions are formed before the fluid leaves the wellhead. 2.2.3 Other Factors Affecting the Stability of Emulsions Other factors that can affect the stability of emulsions are: Viscosity Specific gravity Water percentage Total dissolved solids Age of emulsion Each of these is described in this section. 2.2.3.1 Viscosity The viscosity of a liquid may be thought of as its resistance to flow: the higher the viscosity, the greater the resistance of a liquid to flow. Conversely, the lower the viscosity, the more readily the liquid flows. Often, if a liquid of high viscosity is heated, the viscosity decreases so that the liquid flows more freely. Therefore, heating a crude oil of high viscosity lowers the viscosity and makes it flow easier. An oil of high viscosity requires more time for the water droplets to coalesce and settle out than does an oil of low viscosity. This is because the water droplets cannot move as rapidly through a high viscosity oil as they can through a low viscosity oil. A common example of this may be seen by observing the slow rate at which air bubbles rise in syrup, which has a high viscosity, as compared to the fast rate at which they rise in water, which has a low viscosity. Air bubbles rise, whereas water droplets in oil settle, but the effect is the same. 2-6 Chapter 2: Emulsions 2.2.3.2 Specific Gravity Specific gravity should not be confused with API gravity. The specific gravity of a liquid substance is the weight of a given amount of that liquid at a given temperature compared to the weight of an equal volume of water at the same temperature. For example, if 1 cubic inch of water at 39ºF weighs 1 unit, and 1 cubic inch of another liquid at 39ºF weighs 95 percent of that unit, then the specific gravity of the liquid is 0.95. On the other hand: degrees API = 1415. specific gravity - 131.5 Thus, the water in the example of specific gravity above has an API gravity of 10 degrees, while the liquid with a specific gravity of 0.95 has an API gravity of approximately 17.5 degrees. The difference in specific gravity between the oil and water has a bearing on the stability of the emulsion. The greater the difference, the faster the water can settle. For instance, in a water-inoil emulsion, a heavy oil (one with a high specific gravity and low API gravity) tends to keep water droplets in suspension longer than an oil with low specific gravity and high API gravity. On the other hand, a lighter water such as freshwater does not settle out of any oil as rapidly as salt water because salt water is heavier. The fact that heavier liquids or objects do not stay suspended in a liquid for as long as do lighter liquids or objects can be illustrated by dropping a steel roller bearing and rubber pencil eraser of the same size and shape into a tall glass of water. The steel bearing, which is considerably heavier, goes directly to the bottom, but the lighter rubber eraser sinks slower. Heating the emulsion increases the specific gravity difference between the oil and water (lowering that of oil) in addition to lowering viscosity. 2.2.3.3 Water Percentage A factor that influences, to a certain degree, the tendency of oil and water to emulsify is the relative proportion of oil and water produced. Laboratory tests conducted to determine the influence of oil and water concentrations in emulsions show that emulsification occurs over a wide range of mixtures and that maximum emulsibility is reached at some definite ratio of water to oil. A small percentage of water in oil often emulsifies much more thoroughly and permanently than a large amount. In fact, in many wells producing only small quantities of water, tight emulsions are formed that disappear almost completely if the percentage of water is increased beyond a certain limit. In general, the severity of an emulsion problem usually will diminish when the quantity of water produced by a well approaches or exceeds the quantity of oil produced. 2-7 – Chapter 2: Emulsions 2.2.3.4 Total Dissolved Solids The total dissolved solids (TDS) or salinity of the water also influences settling rates: the heavier the water, the faster the settling. Salinity also influences demulsifier or surfactant partitioning, as high TDS brine may remain clear but relatively freshwater may become cloudy using high RSN demulsifiers. Freshwater emulsions are usually more difficult to treat. 2.2.3.5 Age of Emulsion Crude oil emulsions are systems that are not in stable equilibrium. According to the laws of thermodynamics, such systems change continually in an effort to attain equilibrium. As a result, emulsions increase in stability with age, which generally increases their resistance to dehydration. With time, emulsifying agents can migrate to the dispersed water droplets and coat these droplets completely. Solids (paraffin, clay, etc.) may then coat the emulsified water drops. Age stabilized emulsions may require a much higher chemical rate to treat or even a different chemical from the fresh emulsion. 2.3 Theories of Demulsificati on There are many theories that have been advanced regarding the problem of resolving crude oil emulsions. Unfortunately, these are as diverse as the emulsions they concern, and no one theory is equally applicable in all emulsions. 2.3.1 Reverse Phase In some cases, the breaking of emulsions has been based on the theory that the addition of a reagent, which would produce an oil-in-water emulsion, will break a water-in-oil emulsion by attempting to reverse the phases; and that in so acting, the intermediate condition of complete demulsification will be accomplished. Though this may sometimes be true, it is not always the case. 2 . 3 . 2 Ri gi d Film There is one school of thought that the emulsion-breaking reagent may have the action of making the interfacial film rigid or to convert it from a plastic, somewhat distensible envelope to a glasslike one that has a relatively low coefficient of expansion. When the enclosed water is expanded by heating, the envelope is shattered and the emulsion is broken. To extend this suggestion and assume that the reagent has not only the power of making the film rigid, but actually of contracting it slightly is to supply an explanation of the efficacy of such reagents in the absence of heat. 2-8 – Chapter 2: Emulsions 2.3.3 pH Other schools of thought postulate that the emulsifier is rendered inactive by the addition of the demulsifier through neutralization, change in pH, or loss of solubility. Reverse emulsions especially may be treated by charge neutralization (most reverse emulsion breakers are cationic) or pH change. Most regular emulsions are treated with nonionics. 2 . 3 . 4 E l e c tr onic Cha r ge Still others believe that the emulsifying agents are polar bodies and function because of their electronic charges, and any disturbance of these charges by electron carrying molecules will result in breaking the emulsion. This is especially applicable to reverse emulsions. 2.3.5 Temperature Another possible explanation of the great effect of small temperature increases in some cases is that such added heat is sufficient to cause a change of state in the film ( i.e., converts it from a solid to a liquid and thereby affects its stability greatly). Likewise, the effects of reagents in the absence of added heat have been asserted to be dependent on their power to cause such a change of state in the substance comprising the film, thereby dissolving it from the interface. 2 . 3 . 6 S ur f a c e Te ns i on The theory that petroleum emulsion breaking is caused by a reduction in surface tension is probably the most common. This phenomenon is often referred to without any suggestion as to what constituent is having its surface tension lowered. It is likewise generally predicated on a two-component system, whereas petroleum emulsions are definitely three-component systems. The reagents used may have the incidental effect of reducing the surface tension of either the water or the oil or both, but it is not identical with predicting any emulsion resolution on such reduction as a cause. In any case, the most widely accepted general explanation is that the interfacial surface between the dispersed component and the continuous component is modified in some manner. It is generally recognized that the liquid having the greater surface tension will form the inner, or dispersed, phase. Hence, a change in the surface tension of either component could result in resolution, provided that the surface tension lowering is stopped short of the point of reversing the emulsion. 2-9 – Chapter 2: Emulsions 2.4 Treating Me thods The factors involved in treating water-in-oil emulsions include: 1. Breaking the film surrounding the small water droplets and coalescing the droplets to produce larger drops. 2. Settling the water drops during or after their coalescence. Theoretically, all emulsions separate into oil and water if allowed to settle for an unlimited time. Much of the water produced with petroleum does separate without the assistance of heat, chemicals or other devices. However, the small water droplets in water-in-oil emulsions are usually surrounded by a tough film that gives the appearance of a plastic wrap when viewed under a microscope. This film resists being broken, and until the film is broken, the water droplets do not merge together into coalescence (at least in any reasonable length of time). The higher the viscosity of a water-in-oil emulsion, the slower is the settling rate of the water in it. Thus, if the emulsion is at a low temperature and its viscosity is high, the separation of water from the oil is slow. Also, the smaller the water droplets are in the oil, the longer it takes for them to separate out. In addition, if the difference in gravity between the oil and the water in the emulsion is not great (a small gravity differential), then their separation is slow. All the various treating practices are directed at: Increasing the size of the water droplets. Increasing the gravity difference between the water and oil. Decreasing the viscosity of the oil. Therefore, heat, electricity, mechanical devices, chemicals, and various combinations of them are normally required to cause the film around the water droplet to break and allow coalescence, resulting in improved dehydration. It should also be emphasized that no two oil field emulsions are alike. The procedures used to treat the emulsion produced from one field almost never work as well on an emulsion from a different field. In fact, the emulsion produced from individual wells within the same field sometimes varies. Further, the characteristics of the emulsion produced by a well probably change over a long period of time in the productive life of the well. This often means that some change in treating methods may have to be made if treating is to remain effective. It is seldom possible to establish a specific treating program at the beginning and expect it to be adequate throughout the life of the field. Therefore, the emulsion should be tested frequently and changes implemented as they become necessary. 2-10 – Chapter 2: Emulsions It should be noted that demulsifiers do not stop working overnight unless there is a mechanical problem, chemical contamination such as rain water getting in the tank, or some other change in the field such as acidizing, fracturing, batch treating for corrosion, etc. One form of chemically induced emulsion is demulsifier overtreatment. Demulsifier overtreatment may be indicated by an inability to break the emulsion with a slugging compound during centrifugation (grindout). Also, the appearance of the oil will be different (hazy and possibly showing a slight white foam). The possibility of demulsifier overtreatment may be investigated by verifying pump rates. Demulsifier overtreatment situations may be corrected by turning off the demulsifier pump and sending the overtreated oil back through the system or, in some cases, by washing the overtreated oil with brine. 2 .4. 1 Appl ic a tion of He at Heat alone does not cause an emulsion to break down, except in rare instances. Usually the application of heat is an auxiliary process to reduce the viscosity of the emulsion and allow the water to fall through the oil faster. Indeed, if possible, heat is eliminated entirely from the treating process. Where it is necessary to use heat, one of the many varieties of heaters is used. All emulsion heaters fall into one of two general types: Direct heaters Indirect heaters At present, most treating plants do not employ heaters that are separate from other treating vessels. The heater is usually an integral part of a single treating vessel in which heating and treating are both accomplished. 2.4.1.1 Direct Heaters In a direct heater, an emulsion comes in direct contact with the firebox, or heating element. In general, direct heaters are used to heat non-corrosive emulsions that are under comparatively low pressure. Direct heaters, when operating under proper conditions, are the most efficient type of heater. The efficiency of a heater is determined by figuring out how much gas the heater burned to heat up how many barrels of emulsion to the desired temperature. Four basic types of direct heaters are used in the field: Tubular heaters Fluid-jacket heaters Internal firebox heaters Volume or jug-type heaters 2-11 – Chapter 2: Emulsions 2.4.1.2 Indirect Heaters An indirect heater consists of three main parts: Body Firebox Flow-tube bundle The firebox and flow-tube bundle may be built into the body but are usually removable for easy cleaning, inspection, and replacement. Heat from the firebox is transferred indirectly through a water bath in the body of the vessel to the emulsion being treated in the flow-tube bundle. An indirect heater is less hazardous to operate than a direct heater because the fire does not touch the flow tubes. Because the flow-tube bundle is not warmed by direct heat, the temperature of any flow tube cannot be higher than the temperature of the water bath surrounding it. Also, hot spots do not form in the flow-tube bundle and crack the tubes because the temperature of the water bath is controlled by a thermostat. The relatively low, even temperature of the water bath further minimizes salt and scale deposits. Tube failure is not as likely as in direct heating because many deteriorating effects are held to a minimum. In addition, the oil or emulsion is not in contact with the open flame in the firebox should a failure occur. 2.4.2 Application of Electricity The electrical process of dehydration has been used successfully in various oil producing areas for many years. Since the first electric dehydrator (Chem-Electric) was installed in 1909, many improvements in design and operation have been made. However, the principle of electric dehydration has remained unchanged. The electric field disturbs the surface tension of each drop, probably by causing polar molecules to reorient themselves. The reorientation weakens the film about each drop because the polar molecules are no longer concentrated at its surface. In addition, there is a mutual attraction of adjacent emulsion particles that are given induced charges by the applied electrical field. This causes them to have a tendency to line up along the electrostatic lines of force with opposite charged portions of adjacent particles in close proximity to one another. Since the film is no longer stable, the adjacent drops can now coalesce freely. In this way, the drops grow in size until they are large enough to settle out of the oil by gravity. The addition of heat and chemicals is not an actual function of the electric process of treating emulsions. However, it is usually necessary to add these to accelerate coalescence of the water and thus increase the capacity of the unit and make it more efficient. Also, demulsifiers help prevent interface pad buildup. Any significant emulsion pad in a chem-electric is not acceptable because the emulsion pad will short-out the grids resulting in failure to dehydrate the oil (wet oil). On the next page are diagrams of electrostatic coalescer treaters: one with and one without heat. 2-12 – Chapter 2: Emulsions Typical TriVolt AC Electrostatic Coalescer Courtesy of NATCO Group Dual Polarity(r) Electrostatic Treater with Firetubes Courtesy of NATCO Group 2-13 – Chapter 2: Emulsions 2.4.3 Mechanical Devices Gravity differential is the difference in the specific gravity of water and oil. In most cases, water weighs more than oil and therefore settles to the bottom of a tank containing both; that is, since the specific gravity of water is higher than that of oil, water eventually settles out. This scientific principle forms the basis for all treating procedures. All operations involving chemicals, heat, electricity and mechanical devices are designed to prepare the oil-water mixture for the settling step by speeding up the settling process. However, with certain emulsions, settling alone is sufficient to separate the oil and water. In other cases, heat and chemicals must be added prior to the settling stage. There are four main mechanical devices used for water settling: 1. Settling tank 2. Skim pit 3. Gunbarrel or wash tank 4. Free-water knockout 2-14 – Chapter 2: Emulsions 2.4.3.1 Settling Tank In the early days of emulsion treating, the basic vessel was a settling tank. (See Figure 2.) In many older fields, a number of these tanks are still in use. The emulsion entering the tank may come from the wellhead, a separator, or even some type of heater treater. The well fluids are distributed into the bottom of the tank by means of a distributor, or spreader. The spreader shown is simply four sections of slotted pipe radiating from a four-way tee. The ends of the slotted pipe are plugged so that all the fluids leave the pipe through the slots and are well dispersed over the tank bottom. This simple hookup does not provide for conserving the light ends of the crude. Therefore, the lighter fractions of the produced oil tend to escape, decreasing the gravity and volume of the oil. Nevertheless, a simple settling tank often proves adequate when economy is the prime factor. 2.4.3.2 Skim Pit Conductor Inlet From Field Oil Outlet W ater Siphon Oil W at e r Distribution Rack Pipe - Slotted & Plugged on Ends A skim pit is simply an earthen pit (now generally lined with concrete) into which large volumes of well fluids are produced. Only a fraction of this well fluid is oil, which rises to the surface of the water and is skimmed off by a series of baffles as the water flows across the pit. Although the skim pit represents a final effort to extend the economic life of wells, significant quantities of oil are recovered in this manner. Environmental concerns and opportunities for contamination of the produced fluids have dramatically reduced the use of the skim pit. 2-15 – Chapter 2: Emulsions 2.4.3.3 Gun Barrel or Wash Tank A wash tank, or gun barrel as it is more commonly known, is a settling tank that is fitted with an internal or external boot, or flume. Although gun barrels are not employed to the extent now that they once were, many are still in use. They are worth studying in part because certain principles of breaking emulsions can be observed by a description of gun barrels. In general, gun barrels are composed of five principle parts, each of which serves one or more specific purposes. (See Figure 3.) 2-16 Gas Equalizer Gas Out Emulsion From Field Oil Out Clean Oil Emulsion Conductor Pipe (Downcomer) W ater W ater Water Out Spreader – Chapter 2: Emulsions 1. The inlet line is the pipe that conducts the emulsion (water and oil) from the oil and gas separator to the gun barrel. 2. The conductor pipe (also known as the boot, flume, downcomer, or stack) is the large pipe through which the emulsion passes before entering the bottom of the gun barrel. The boot may be mounted either inside or outside the tank, and serves three main purposes: a. Gas separates from the emulsion inside the boot, and thus, turbulence is reduced within the body of the gun barrel. b. It serves as a surge tank to prevent slugs of emulsion from being injected into the gun barrel. c. It spreads the emulsion more evenly throughout the water wash by means of a spreader, or apron, which is attached to the bottom of the boot. 3. The body, or tank, holds the water wash (or water layer), emulsion, and clean oil layers. This allows time for the oil and water to separate. 4. The water outlet (also called the water leg, outside siphon, or grasshopper) serves two purposes: a. It provides an outlet for the water that has separated from the emulsion. b. It regulates the amount of water held in the gun barrel. 5. The oil outlet line conducts the clean oil from the gun barrel to the storage tanks. The majority of gun barrels have several other parts, such as gas equalizers between the tank and conductor pipes, gas lines, bleeder line, and gauge glasses. The oil and water interface may be seen through the gauge glass. The principles on which the gun barrel operates are best seen by tracing the path of the emulsion through it and describing what happens in each step. (Refer to Figure 3.) Assume that settling is being used as the sole means of separation of the water and oil and that no heat or chemical is added. (Although often chemicals are injected and a heater is installed in the system before the emulsion reaches the gun barrel.) As the emulsion enters the conductor pipe from the inlet, it is subjected only to atmospheric pressure. Since it is necessary to exert pressure on the oil and gas separator, which is located in the emulsion stream ahead of the gun barrel, some gas comes out of solution with the decrease in pressure as the emulsion enters the gun barrel. This gas is carried out through a gas outlet line to be vented, or to a gas gathering system. Only liquid flows down the conductor pipe to enter the gun barrel near the bottom. 2-17 – Chapter 2: Emulsions A spreader is placed on the bottom of the conductor pipe to spread the emulsion out so that it is distributed through the water wash. If the spreader was not there, the emulsion would channel through the free water held in the gun barrel in one large column. The spreader is usually placed about two feet off the bottom of the vessel. This depth immerses the spreader as deeply as possible in the water, yet keeps it above the sludge that may accumulate in the bottom of the tank. The diameter of the spreader depends on the size of the gun barrel; it is usually from about 40 to 70 percent of the diameter of the tank, but some are smaller. Some emulsion breaking occurs as the emulsion comes in contact with the surface of the spreader and flows from the center to the outside rim of the spreader. Spreaders are designed so that the emulsion emerges from them in very small streams. As the streams of emulsion rise through the free water, some emulsion breaking occurs by the close contact of the emulsion and free water. Many of the water droplets are washed out of the oil, allowing clean oil to continue to rise. Above the free water held in the gun barrel are two liquid layers, the top layer containing clean oil and the next layer containing emulsion. These layers are not clearly defined, but blend into each other. As the emulsion rises fairly rapidly through the free water due to the difference in specific gravity of the two liquids (oil being lighter than water), it goes into the layer of emulsion already present above the free-water layer. In the emulsion layer, the rate of travel is slower, and the remaining water and solids settle out. Oil, being lighter than emulsion, rises to the top and exits through the oil outlet to the storage tanks. In summary, the action that occurs in the gun barrel to separate oil and water is divided into two main parts: 1. Washing — The washing is done in the free-water layer. 2. Settling — Settling occurs in the emulsion layer. Since not all emulsions are alike, no set pattern on the amount of free water that should be held in a gun barrel can be established. For instance, washing has little or no effect on certain emulsions; therefore, in such cases a very small amount of free water in the tank is all that is necessary. On the other hand, some emulsions completely break down by washing; therefore, it is advantageous to have a large amount of free water in the gun barrel. When selecting a demulsifier for a gun barrel system, it is especially important to select the product that shows the lowest BS in the grindout. This will reduce the potential for accumulation of an interface pad. 2-18 – Chapter 2: Emulsions 2.4.3.4 Free-Water Knockout (FWKO) Free water is produced when oil settles within five minutes while the well fluids are stationary in a settling space within a vessel. Free water, then, is not part of the emulsion and may be readily separated by the force of gravity alone. Free-water removal prevents overloading the heating and treating plant. For instance, consider that it takes about three and one half times more heat to raise the temperature of water than oil. Therefore, if most or all of the free water is removed first, then substantial savings in the fuel needed to fire the heater can be made. A free-water knockout is a vessel that is used to remove excessive amounts of free water in the flowlines ahead of the treating plant. While there are many different configurations, free-water knockouts are either two-phase or three-phase in design. A two-phase FWKO is designed such that only the free water separates from the oil or emulsion. A three-phase FWKO separates free water and gas from the oil or emulsion. (See Figure 4.) In general, a free-water knockout is simply a vessel that provides a space for free water to settle out of an emulsion. Sometimes filter material is installed in the FWKO to aid in removing tiny droplets of oil or emulsion that may be entrained in the water as it passes through the filter. The free water is drawn off the bottom of the unit, and the emulsion or oil passes out the top to the treating system. Thus, all free water is removed, and only the emulsion is handled by the heating or treating system. 2-19 Gas Out Baffles Emulsion Inlet Emulsion Out Water Water Out – Chapter 2: Emulsions 2.4.4 Floating Production, Storage, and Offloading (FPSO) System Traditionally, oil fields have been produced by building a platform on the site after appraisal drilling operations had been carried out. However, across the world, oil and gas is being found and produced in ever-deeper waters. Advancements in technology have meant that smaller oil fields, where it would not have been economically viable to build a platform, are now able to be developed by the use of a cheaper option — a Floating Production, Storage, and Offloading vessel, or FPSO for short. The offshore oil and gas industry has been using floating production, storage, and offloading systems, or FPSOs, since the mid-1970s. They can offer two significant advantages over fixed production platforms: A fixed installation may not be technically feasible in a particularly challenging location where a floating unit would offer the best solution. This is the case in remote offshore locations where deep water, strong ocean currents, and harsh weather conditions may occur, or where export pipelines are difficult to install or uneconomic to run. Floating systems are also a cost-effective solution for developing smaller, satellite or marginal fields in shallower water as they can be floated off when reservoirs are depleted, and re-used elsewhere. The benefits of “recycling” such facilities are not just economic but also environmental, particularly for marginal fields where the production facilities may only be required for a few years. An FPSO is similar in appearance to a ship but is designed quite differently and carries on board all the necessary production and processing facilities normally associated with a fixed oil and gas platform. The main difference between an FPSO and a fixed platform is that the produced oil is stored in holding tanks situated in the hull of the vessel. Every few days an oil tanker comes along and links to the FPSO, the oil is transferred and then taken by the tanker to a refinery. 2-20 – Chapter 2: Emulsions The FPSO shown below is the Schiehallion, run by BP and situated North of Scotland in the North Atlantic. The FPSO is moored permanently on location over the reservoir. Where weather conditions can be extreme, most vessels have a central mooring arrangement located within the hull in a turret that allows them to rotate freely around the point of mooring in response to shifting weather direction. This is known as weathervaning and allows the vessel’s bow always to point into the prevailing wind and currents, minimizing the impact of nature’s forces. In countries with more benign weather, such an arrangement may not be required, and the vessel is kept on station by an array of moorings and anchors, known as a spread-moored system. The hydrocarbons treated on an FPSO are produced through wells that are located on the seabed. Untreated liquids are brought to the surface via subsea equipment on the sea floor including valves at the well and a manifold to connect several wells together into one flowline. These flowlines are then linked to the vessel by flexible risers that pass from the seabed to the floating facility at the surface. They must be flexible to accommodate the heaving motion of the vessel above and be very resistant to fatigue. 2-21 – Chapter 2: Emulsions There are currently 15 FPSO units operating on the UK Continental Shelf and 70 worldwide. The diagram below shows the Foinaven FPSO, situated in the North Atlantic, North of Scotland. The oilfields lie in a water depth of between 400 and 600 metres. (Britain’s tallest building and largest offshore platform are shown for scale.) The diagram shows that the oil is produced via a manifold, which passes through rigid flowlines and then flexible risers and onto the FPSO. 2.4.4.1 FPSO Processes and Factors Affecting Bottle Tests In general, topside separation process systems are fairly similar to that of fixed offshore installations. Anything from a two- to a four-stage separation system is common, with possibly one or two trains. Due to the limited capacity of the topside system, residence times can be short depending on production. The main difference between an FPSO system and a typical fixed installation system is that the crude is sent to storage tanks and not to an export pipeline. Often the storage tanks can be used for further, longer-term separation if the vessel has the facility to remove water from the bottom of the tanks. 2-22 – Chapter 2: Emulsions Factors to consider in the Bottle Tests are: If water can be removed from the storage tanks then it is not as crucial to remove all the water in the topside process. In this case, slower-acting but more complete water drop may be required, and the test may be amended to include the longer residence time in the storage tanks (usually at reduced temperatures). If water removal is not possible from the storage tanks, then the fluids going down to the storage tanks will contain the same amount of water as the crude exported to the tanker. In this case, a fast-acting water dropper will be required to suit the expected very short residence times in the separators. Crude dehydration will also have to match export specifications. Water quality is a very important factor when Bottle Testing on an FPSO. Separated water is either re-injected into the formation or pumped overboard, and as such, the oil-in-water specifications will be strict and good water quality in the Bottle Tests is essential. It is often the case that the system dynamics and the movement of the vessel (and hence separators) in bad weather require a tighter than normal interface to avoid bad water quality in the system. It is important not to have a “dusty” interface when swirling the bottle as this could result in high oilin-water figures in the system, particularly in bad weather conditions. 2.4.5 Chemicals Under proper conditions, emulsions are resolved quickly and effectively by chemicals synthesized to have demulsifying properties. To break an emulsion chemically, the chemical must be carried to the interface of the emulsified water and the surrounding oil. In this action, it is believed that the chemical powers the interfacial tension of the oil and water, allowing the dispersed particles to coalesce into larger drops, which then separate from the oil. The resolution of emulsions by chemical means has a wide range of application; it is equally adaptable to either large- or small-scale operations and has a high degree of flexibility. Additionally, chemical treatment allows facilities to be proportioned to the volume of oil treated. Therefore, the installation of a large capacity plant during the period of flush production does not penalize the operator unduly by increasing his treating costs later when the quantity of emulsion to be treated has declined. Chemical dehydration requires only a low initial investment in plant equipment, and operating costs are not high. 2-23 – Chapter 2: Emulsions The success of treating emulsified oil depends on: An adequate quantity of the most effective chemical. Sufficient agitation to cause thorough mixing of the chemical with the emulsion. Where necessary, the addition of heat to facilitate breaking of the emulsion. Cold treating may be possible if ambient temperature is above the paraffin cloud point, or if working with a frozen or icy emulsion, the emulsion is first melted. Cold treating usually requires considerably more demulsifier than treating with heat. There is usually an economically effective ratio of chemical to heat, as well as a practical one. Proper handling and separation of the gas before settling. Sufficient time to permit settling of the released water. 2.4.5.1 Chemical Injection Points To obtain uniform distribution of the demulsifier and maximum chemical action with minimum chemical consumption, the demulsifier should be introduced where the subsequent flow through the system will provide optimum agitation. In general, best treating results are obtained by introducing chemical before the well fluid enters the gas separator, preferably at the wellhead or into the flowline as close to the wellhead as possible. In this way, use is made of the agitation in the flowline and the equipment between the well and the separation equipment. Injecting the treating chemical into the well fluid after it has passed through the separator usually results in excessive use of chemical because the amount of agitation in the system beyond the separator is insufficient to be fully effective. Where flowlines from several wells are manifolded into a header and the emulsion from the different wells are relatively uniform, it is desirable to introduce the chemical directly into the header. Where one well is producing most of the emulsified oil, it is often practical to inject the chemical necessary for all the wells into the flowline near the wellhead of the offending well. Of course, other provisions for injecting chemical must be made if the well producing most of the emulsion is shut-in. 2.4.5.2 Selection of Chemicals — The Bottle Test The Bottle Test is performed to assist in the selection of the treating compound that will most effectively break the emulsions from any given well, lease, or field. The Bottle Test results may also be used as an indication of the ratio of treating compound to emulsion, which will be required to achieve salable oil. 2-24 – Chapter 2: Emulsions There are some basic rules that need to be followed in carrying out a proper and informative Bottle Test. The sample used for the Bottle Test must be chemical free and representative of the emulsion to be treated. If possible, always use a composite sample. The sample should be as fresh as possible because rapid aging of some emulsions affects their susceptibility to treating. The same conditions of agitation, heat, dosage, and retention time as are found on the lease should be simulated as closely as possible. The system survey should also note any recent changes in the field, chemicals, workovers, etc. The Bottle Test is performed in three separate functions: Ratio Test, Elimination Test, and Confirmation Test. These are described briefly below. For a detailed Bottle Test Procedure see section 2.5. Ratio Test The Ratio Test is the first function of the Bottle Test. Normally, the compound in use for the emulsion is dosed at different levels to determine the proper dosage for treatment. A general rule of thumb is to dose the compound at three levels below and three levels above the existing rate. The Ratio Test prevents wasting time in the Elimination Test by dosing too low (resulting in no treatment) or dosing too high (resulting in too many compounds giving good results and possibly resulting in overtreatment). The Ratio Test is also where the parameters of the Bottle Test are defined. This is where, based on system information, agitation, dosage, heat, and retention time are determined to produce salable oil with the compound in use. An overtreat ratio (three to five times the normal treating rate) indicates if overtreating is a problem. If at all possible, avoid products that overtreat. The low ratio, below the treating rate, exaggerates treating differences and helps select the best product. Elimination Test The second function of the Bottle Test is the Elimination Test. After the test parameters have been determined, the bulk of the testing will be accomplished during the Elimination Test. Instead of dosing one compound at several ratios, many compounds will be dosed at the same ratio. Sometimes, many compounds are dosed at several ratios. The ratio to use will be based on the results of the Ratio Test. The Elimination Test is completed when all the desired compounds have been screened and several promising ones have been identified. Re-emulsification Test After grindouts have been obtained, re-mix the separated emulsions of the best chemical candidates to determine which do not re-emulsify. 2-25 – Chapter 2: Emulsions Confirmation Test The Confirmation Test is the last function of the Bottle Test. The Confirmation Test is nothing more than a Ratio Test with the best compounds identified during the Elimination Test. Several ratios below and several ratios above the dosage that gives salable oil should be run. The results of the Confirmation Test should: Determine the best compound that treats this emulsion to pipeline oil. Indicate the optimum and range of the dosage. Bottle Test Results During the Bottle Test, compounds are evaluated and observations are made on several criteria. These observations are recorded on a Bottle Test Report form for comparison study and as a permanent record of knowledge. Below are the main criteria recorded on the Bottle Test Report for the evaluation of emulsion breakers. 1. Water Drop Water drop is defined to be the water that coalesces and settles to the bottom of the prescription bottle. The relative speed of the breaking of an emulsion is usually indicated by the speed of water drop. The speed of water drop can be misleading. Sometimes a compound will show rapid initial water drop and then stop before all the water is released. The best water drop is both fast and complete. The importance of the speed of water drop depends on the system treating the emulsion. Generally, as the retention time is increased, the importance of speed is decreased. It is generally advisable not to select a product that drops water much faster than the system residence time. 2. BS&W Content of Oil BS&W stands for basic sediment and water. Basic sediment is usually unresolved emulsion but can also include organic and inorganic solids. BS&W can be distinguished in crude oil by a trained and experienced Bottle Tester using the naked eye. As the BS&W content decreases, the deeper the color and brighter, or polished looking, the oil layer becomes. The best and most accurate way to measure BS&W content is to perform a thief grindout and slug grindout on the oil. The thief grindout measurement tells how well the emulsion is resolving and how complete is the water release or dehydration. The slug grindout tells if there is any secondary emulsion in the oil. Secondary emulsion is a new term that will be defined and distinguished from normal, or primary emulsion, as follows: a. Primary Emulsion The BS that is thrown down on centrifuging a sample of crude oil without the addition of an excess (slug) amount of treating chemical. Primary emulsion is thrown down as a more or less well defined layer. 2-26 – Chapter 2: Emulsions b. Secondary Emulsion The additional amount of BS which is present in the grindout tube which did not pack down on centrifuging and which on treatment with an excess (slug) of chemical is broken down to oil and water. The presence of secondary emulsion results in the water reading of the slug grindout to be larger than the sum of the BS and water readings of the unslugged grindout. Generally, the best compound will be the one that has the lowest slug grindout with the least amount of BS in the unslugged grindout. If there is a question whether the sediment is paraffin or emulsion, the centrifuge tube may be heated. If the sediment separates with heating to show water and oil, it is emulsion. If the sediment melts and no water appears, tilt the tube. Paraffin will resolidify along the side of the tube. 3. Interface In the ideal treatment of crude oil emulsions, the oil-water interface should be a sharp, clean line without any web or sludge. Presence of a considerable amount of sludge or web is undesirable. In a treating plant, this foreign material will eventually go to stock and be reported as BS. Trace amounts of web or sludge seen in the Bottle Test, however, may disappear or treat out in the treating plant. Foreign materials at the interface can often be seen through visual observations and are recorded for reference. Sometimes, the condition of the interface is not easily seen and needs a more accurate method of evaluation. The composite grindout is the tool used to determine the quality of the oil contained between the oil-water line and the level at which the thief grindout was taken. Some compounds are referred to as “sludgers.” This means that they give good thief grindouts but make sludge of water, BS, or both at the interface. These compounds may yield clean oil for a time, but eventually a pad will build and grow at the interface, spilling over to stock and causing bad oil. The composite grindout will reveal this potential problem. 4. Water Quality During Bottle Tests, water quality is noted and recorded for reference. Concern for the environment, injection well plugging, formation damage, and increased treating cost are a few reasons why water quality is important in treating regular emulsions. Although most production facilities have water treatment systems, it is important not to add to or create water problems with regular emulsion breakers. Selecting an emulsion breaker compound that produces clean oil and clean water is the ultimate goal. High RSN demulsifiers or wetting agents (surfactants) can cause cloudy water by dispersing oil in the water phase, especially in freshwater. In high TDS water, higher RSN products may be used without oil-in-water problems. 2-27 – Chapter 2: Emulsions 5. Treating Range Compounds with the widest treating range that yields good emulsion treatment are preferred, but not always the best for a certain application. Wide treating range products are better able to handle fluctuations in product rates, system upsets, and temperature changes and are easier to introduce into a treating plant. Wide treating range compounds also help overcome the tendency of field operators to increase dosages when system upsets occur. If this happens and the compound goes into an overtreat condition, this attempted solution can make the problem worse than the original problem. Testing on a composite sample over several days is recommended to insure consistent demulsification. If you select an emulsion breaker based on samples from one well, there is a chance the product may not be effective for the entire field. The treating system may dictate the importance of one factor to be weighted more heavily than another; but all of these criteria should be considered when evaluating emulsion breakers by the Bottle Test. The Bottle Test is not an exact science, only a tool to aid in the selection of emulsion breaker compounds. It is a static test performed on a dynamic system and cannot duplicate the true fluids. Nevertheless, the Bottle Test remains the industry standard for emulsion breaker evaluation. 2.5 Field Characterization and Application 2.5.1 The Bottle Test The following describes the steps in the Bottle Test in detail. 2.5.1.1 Field System Survey This first step is the most important step in doing a regular emulsion breaker Bottle Test right the first time. By going through this procedure, your emulsion breaker Bottle Testing will produce more accurate and meaningful results. Deleting the Field System Survey from your Bottle Test procedure may cause you to make inaccurate or insufficient interpretations of the test results. In a worst case, failure to perform a good survey might ruin your chances at a successful proposal or plant test. To benefit you the most, the Field System Survey should include the following items. (Note: items marked with an asterisk (*) are essential to a successful Bottle Test.) 1. What is the total oil/water production?* 2. How many producing wells are there? 3. What is the recent well test for each well? (an important key) 2-28 – Chapter 2: Emulsions 4. From what zones is the field producing and which ones are creating an oil treating problem? 5. Where is the best place to obtain a good emulsified oil sample? (A few producers, a common header, upstream of a separator, etc.?)* 6. What is the API gravity? 7. What are the chlorides of the produced water (in mg/L as NaCl)? 8. What emulsion breaker is being used in the field?* At what locations? At what ppm? Obtain a small sample of the emulsion breaker for testing. Important: It is illegal to obtain a product sample without the permission of the owner of the supply of product; usually the customer is the owner. 9. Which wells are creating the most difficult oil treating problems?* 10. Is there a flowing pressure problem? A downhole viscosity problem creating a loss in production? 11. What type of treating system is on location? (Vertical treaters, horizontal treaters, FWKOs, gun barrels, etc.?)* 12. Are paraffin or asphaltenes a factor in oil treating?* 13. By what method are the wells being produced? (Gas lift, beam unit, flowing, submersible, etc.?) 14. Identify other types of chemicals being used in the oil field other than the emulsion breaker. Where are they being injected? At what rates (ppm)? 15. Obtain or draw a complete diagram of the field system. (an important key)* Show all lines and vessels (treaters, separators, gunbarrels, Wemcos, etc.). Show all directions of fluid movement. Identify where all chemicals are being injected and at what rates (ppm). 2-29 – Chapter 2: Emulsions 16. What is the retention time of the system? (an important key)* 17. What is the temperature of the oil treating system? (an important key)* 18. Is the system affected by secondary or tertiary recovery? 19. Is the system affected by slop oil, pit oil, squeezes (scale, paraffin, corrosion, emulsion breakers), mud-acid flowbacks, solvent soaks, acid jobs, corrosion batch treatments, etc.?* 20. Are solids a problem? (Iron sulfide, sand, scale, asphaltenes, paraffin, etc.?)* 21. What kind of recirculation occurs? (Tank bottoms, pits, Wemco skimmings, platform deck drains, sumps, etc.?) 22. Finally, record any comments of customer and contract personnel that pertain to the current performance of the oil treating system, whether they are mechanical or chemical related. 2.5.1.2 Ratio Test 1. Complete all known information on the Bottle Test Report. If you should have a problem interpreting your Bottle Test results, other OFC personnel can assist you. 2. Obtain a chemical-free sample of crude oil emulsion. 3. Drain all free water from the sample. Add free water back to each bottle to give a water/oil ratio that reflects the production. Never exceed a total water amount in the bottle of 60%. This free water will closely mirror results of the emulsion breaker treatment in the initial separation and indicate potential water quality problems. 4. Shake the sample container to obtain a homogeneous sample for a grindout. Centrifuge two samples: one chemical free, the other slugged with a few drops of cut EC2003A. Straight EC2003A is not normally used, as an overtreat can sometimes occur. EC2003A is usually cut back with a solvent (R-3533, R-3320, etc.) to 50:50, 30:70, etc., depending on your experience and area. DO NOT USE GASOLINE as a solvent in any emulsion breaker test. Gasoline contains emulsion breakers! 5. Fill seven bottles to the 100 ml mark with freshly collected crude oil emulsion and invert several times, so that the bottles will be coated with an emulsion film. The seventh bottle will be the blank. 6. Make a 10% solution of the formula being used in the system only when the API° gravity of the oil is less than 18° gravity, otherwise use the drum strength solution, and pipette chemical concentrations: three below and three above the existing rate. Example: if the chemical concentration in the system is presently 120 ppm, the concentrations of 50, 80, 110, 150, 180 2-30 – Chapter 2: Emulsions and 200 ppm plus the blank could be evaluated. Note: Any 10% emulsion breakers should be made with a PCC solvent such as Lactene, Xylene, etc. DO NOT USE GASOLINE. 7. Cold agitate bottles to ensure adequate dispersion of chemical in the emulsified oil. The amount of agitation is determined from the field system survey and the amount of agitation occurring in the system between the point of demulsifier injection and the treating vessels. Cold agitation means at the temperature of the fluid taken at the demulsifier injection point or at the inlet to the treating vessels. Example: if the fluid temperature at the injection point is 60°F, then agitate at 60°F; if the temperature at the injection point is 140°F, cold agitation should be at 140°F. 8. If the emulsion requires heat for treatment, place the bottles in a water bath adjusted to the treating vessel temperature. 9. Every few minutes examine the bottles carefully and record water drop, the presence of a BS layer, and general appearance on the Bottle Test Report. A rule of thumb for the time period between readings: only take readings if there has been a significant change in water drop in one or more bottles since the previous reading. 10. After some time, based on many bottles showing good water drop and the information gathered from the Field System Survey, agitate a second time (hot agitation). Cold treating may also see a second agitation, depending upon your Field System Survey. 11. After hot agitation, record water drop, presence of a BS layer, and general appearance on the Bottle Test sheet. 12. After maximum water drop is achieved, based on the grindout from Step 11 above, perform the Thiefing Grindouts on the treated crude oil samples: a. Fill a centrifuge tube to the 50% mark with some type of suitable hydrocarbon solvent (Stoddard Solvent, White Gas, Toluene, R-3533, etc.). DO NOT USE GASOLINE. b. Select the bottle with the most water drop volume. Adjust a thief pipette so that an oil sample can be taken from a point 15 ml above the water level in this bottle. Use this setting for thiefing all the bottles in this test. Do not adjust the thief level for each bottle. c. Draw oil from the first bottle and fill the centrifuge tube to the 100% mark. Mix the oil and solvent in the tube by shaking. If paraffin is present, heat the tubes if necessary to 120-150°F. Spin for three to five minutes in the centrifuge. 2-31 – Chapter 2: Emulsions d. Continue this process with all of the treated samples. e. Record the water and remaining BS in their proper columns on the Bottle Test Report in the section labeled “Thief Grind Out.” 13. Run an excess chemical grindout on each centrifuge tube: a. Add two to three drops of cut EC2003A to each centrifuge tube and agitate each tube vigorously, making certain than any BS layer is broken up. b. Heat the tubes if paraffin is present. c. Centrifuge three to five minutes and record the results in the section of the Bottle Test Report labeled “Slug” (“Excess” on some forms). d. Record any presence of unbroken BS, the color of water at the bottom of the tube, and any solids at the bottom of the tube. 14. Some systems require a Composite Grind Out for meaningful data. Field experience will tell you if this needs to be done. This procedure requires you to: a. Extract, with a pipette, all the free water at the bottom of each bottle. b. Fill centrifuge tubes to the 50% mark with a proper hydrocarbon solvent. c. Recap each bottle and shake vigorously to make the remaining oil-water emulsion homogenous. d. Immediately pour a sample from each bottle into the centrifuge tubes, filling to the 100% mark. e. Centrifuge, as in the Thief Grind Out, and record results for water and BS in the section of the Bottle Test Report labeled “Composite Grind Out.” f. Perform an excess chemical grindout with cut EC2003A, as in the Thief Grind Out and record. 2-32 – Chapter 2: Emulsions 2.5.1.3 Elimination Test Procedure 1. Select the OFC formulas that your past experience indicates to be of value for the type of emulsified crude in question. a. Take this opportunity to test all new OFC compounds for your records. b. Always include the emulsion breaker used in the field you are testing, whether it is an OFC product or one of the competitor’s. The emulsion breaker Bottle Test is not valid without it! 2. Follow the same procedure as in the Ratio Test except that now you will inject several products at one ratio rather than one product at several ratios. The ratio to be used will be based on your observations in the Ratio Test. In the Elimination Test, you want to find a product that performs better than the one currently in the system; therefore, the Elimination Test should be run at a ratio that did not quite produce pipeline oil in the Ratio Test. Example: in the Ratio Test the product currently in the system was treated at 60, 90, 120, 150, 180, and 210 ppm. Pipeline oil in the system is <1.0% BS&W. Thief grind out were less than 1.0% at the ratios 150, 180, and 210 ppm. The Elimination Test should be run at a ratio less than 150 ppm, perhaps at 120 ppm. 3. It is strongly recommended that two or three ratios of each product be tested, rather than just one, if time permits. A two or three Ratio Test of each product will give meaningful information regarding treating range. 4. From the above procedures, the best three or more OFC emulsion breakers plus the emulsion breaker currently being used in the field should be chosen for further evaluation in the Confirmation Test. 2-33 – Chapter 2: Emulsions 2.5.1.4 Confirmation Test Procedure 1. The Confirmation Test is run exactly like the Ratio and Elimination Tests, except that only the OFC products that performed the best in the Elimination Test are used. a. The test is run at two ratios below and one ratio above the ratios used in the Elimination Test. b. The purpose of this test is to remove all doubts as to which OFC emulsion breaker is right for the emulsified crude in question. c. Again, this test must also include the emulsion breaker currently used in the field. 2. After the Ratio Test, Elimination Test, and Confirmation Test have been completed, the OFC product best for the system is determined by considering these factors: a. Relative speed of the breaking of the emulsion, which is usually indicated by the speed of water drop. b. The color and brilliance of the oil layer. c. The color and brilliance of emulsion/oil remaining on the top section of the bottle. d. The appearance of the oil-water interface. The best performance is usually indicated by a clean, smooth break and the absence of any so-called “webby” or “raggy” material. e. The ability to most nearly produce treated oil to pipeline specifications. This means the lowest BS&W results both before and after slugging with EC2003A. f. The product that drops the cleanest water. Cloudy, dirty water produced by an emulsion breaker in the Bottle Test may indicate that the product would cause overboard or water injection problems. 2-34 – Chapter 2: Emulsions 2.5.1.5 Bottle Testing Systems Making Less than 50 bbls Oil per Day (BOPD) 1. When Bottle Testing Systems making less than 50 BOPD, the ratios used in the Bottle Testing must be adjusted to account for the fact that most chemical injection pumps will not pump at a rate less than one quart per day. 2. First, determine the amount of oil and water produced. 3. Assume that the chemical pump can pump a minimum of 1 quart per day effectively. 4. Calculate the ratios to use in each test based on the use of 1 quart per day of product. Example: the lease makes 15 BOPD and 15 BWPD. In your Ratio Test, you would use the equivalent of one quart of chemical per 15 barrels of oil, one quart of chemical per 20 barrels of oil, one quart per 25 barrels of oil, etc.; or about 400 ppm, 300 ppm, and 240 ppm respectively. These ratios are calculated using the formula: Ratio, ppm = 5,925 / bbl oil 5. This adjustment allows you to simulate the system and conduct your testing under more realistic conditions. The emulsion breakers used under these conditions usually are either less active or have a wide treating range. 2.5.1.6 Interpretation of Results 1. Speed of Water Drop The speed of water drop can be misleading, because occasionally an emulsion breaker will show rapid initial water drop and then stop before all the water is released. Consider only those emulsion breakers that release all of the water. 2. Color and Brilliance of Oil The color and brilliance of oil in transmitting light is very important and a trained observer can detect the presence of BS&W with the naked eye. Generally, the brilliance and the depth of color increases with the decrease in BS&W content. 3. Oil-Water Interface In the ideal treatment of crude oil emulsions, the oil-water interface is sharp and clean without any web, rag, or sludge present. 2-35 – Chapter 2: Emulsions 4. Water Quality The water at the end of the test should be crystal clear. Cloudy, dirty water produced by an emulsion breaker in the Bottle Test may indicate that the product would cause quality or oil carry-over problems in the system. 5. Low BS&W The lowest BS&W readings in the grindouts, along with the water quality and rapidity of water drop in the allocated treating time, will indicate which of the treating emulsion breakers evaluated would be the initial choice to be plant tested. 6. Additional Factors a. Dispersibility of OFC emulsion breaker in the produced waters. b. Success of selected OFC emulsion breaker at other locations in your area. c. Availability of selected OFC emulsion breaker. d. Cost-effectiveness of selected OFC emulsion breaker. e. Your experience in the area with the type of emulsified crude you are testing. 2 . 5 . 2 Th e F i e l d Tr i a l This working procedure is intended as a detailed guide for personnel who are required to supervise a demulsifier evaluation trial on any type of oil producing facility whether it be onshore or offshore. Because of the wide variety of treating systems, it is beyond the scope of this document to even attempt to provide a procedure that accounts for all possible eventualities which arise during a field trial situation. The intention of this procedure is to direct the worker to find the relevant information that will allow the worker to deal with the particular nature of this type of work. The reader should also familiarize himself with chemical changeover procedures. For BS&W determination method, see Institute of Petroleum method IP 359. 2-36 – Chapter 2: Emulsions 2.5.2.1 Pre-Trial 1. Before travelling to the field, the worker should have a clear objective of what is required of the trial and of the trial chemical. The original Bottle Test Report should be consulted as should the original Bottle Tester who developed the trial product. 2. The worker should be aware of the quantity of chemical submitted for the trial and type of container — i.e., drum or tank — that he should expect to be waiting for him in the field. 3. Any reports relevant to the field to be visited should be consulted as these often give the worker information about the production system and relevant production data. 2.5.2.2 Trial 1. Having arrived in the field, the first important job is to meet the production operations personnel and explain why you are there and what you intend to do. Ask what problems they are encountering in the system and get some history of the problem. Typical problems are high BS&W, high oil in waters (OIW), emulsion pad build-up, etc. Don’t be afraid to ask different operators the same question: there are always different versions of the story. Remember that nobody knows the system as well as the people who operate it, so listen to their advice and requests. Throughout the trial always keep the operators informed of what you are doing and give summaries to operators who are new on shift. 2. Before beginning the trial, you will need the following information from the control room. This is documented in the form of a checklist to help data compilation 3. Armed with this information (the checklist), the worker should have a good appreciation of the production facilities and what is required from the chemical treatment. 4. The worker should now request an operator to show him the entire separation system from wells to export line. Note the number and positions of sample and injection points. Try and find sample points at the inlet and outlet of all the separation vessels. Become familiar with how the chemical injection pump is changed to deliver a different chemical rate and how to measure the rate of delivery. 5. Arrange for the new demulsifier chemical to be installed ready to commence injection. Refer to the chemical changeover procedure. Make sure CHDS sheets are available if required. 6. Normally a day is spent monitoring the performance of the existing chemical in the system and getting a feel for its abilities and limitations. Grind outs should be run out of each vessel. Oil-water interfaces should be checked for emulsion or solids pads. If the worker feels 2-37 – Chapter 2: Emulsions confident with the historical data, or he has knowledge of the system and chemical, then he can commence with the trial. 7. The trial chemical should be initially injected at a rate of 10% above the treat rate of the incumbent chemical. When demulsifiers are changed, there may be an initial increase in residual BS&W in the system following the changeover. The emulsion is being treated with a different chemistry, which can have an unsettling effect initially; this period will not last long if the chemical treats the emulsion well. It will take a little time for the chemical to equilibrate throughout the system, the length of time being dependent on the residence time of the system. Be prepared for this potential, temporary upset; explain to the operators why this occurs. Record the time when treatment with the chemical started. 8. The responsibility of the worker is now to monitor the progress of the trial by taking regular BS&W and OIW measurements. Oil-water interfaces should be monitored periodically throughout the plant trial to ensure no pad of emulsion or solids develop. Most operators take daily BS&W readings of the export line, but this does not give any information about how the individual separators are performing and how efficiently they are removing BS&W. It is better to monitor the BS&W profile of the system by taking regular oil samples throughout the separation system. Given the inlet and outlet BS&W of a separator, it is possible to measure the separator efficiency. For this to be representative samples should be good (i.e., taken from the side of a line, preferably on a vertical section of pipe). Separator efficiency = (Inlet BS&W - Outlet BS&W) x 100% % Inlet BS&W With this sort of information, it is possible to pinpoint areas for mechanical/system improvements. Under certain circumstances, it may enable the worker to defend the performance of the trial chemical, which is often the first thing to come under criticism should BS&W remain high. 9. Depending upon the performance of the trial product, it will be necessary either to increase or decrease the treat-rate of the chemical. The overall objective is to lower the treat-rate of the chemical while maintaining the pipeline specification of the crude. Reduction and increases in treat-rate should be gradual until the worker gets a “feel” for the system and how it will respond. Do not be tempted to make too many changes too soon or all together. 10. Certain system parameters can be changed to try to improve demulsifier efficiency; a combination of these may be required. System Temperature — Generally an increase in treat temperature will improve separation; however, there will always be an upper operational limit. Demulsifier Injection Points — Moving injection points further upstream will give the demulsifier more time to perform. 2-38 – Chapter 2: Emulsions Separator Retention Times — By lowering the crude-water interface it will be possible to increase the crude residence time in the separators. Care must be taken; if the interface is lowered too much, it can result in bad oil-in-water. Always consult the operators before making any system changes. Try to make system/chemical changes during the daylight shift. 11. Each operator will have his own criteria for measuring the success of a trial. Generally, if the trial chemical resolves some operating problem even at a slightly higher treat rate, or if it gives lower BS&W at the same or lower treat-rate, it will have succeeded. Trials usually fail because the crude does not meet pipeline specification or the OIW are bad dispute increases in chemical treatment. In the event of a failed trial, the worker needs to know why it has failed. The operator will request that the trial be stopped. The worker should assist the operators in injecting the old chemical back into the system and help achieve steady conditions. 2.5.2.3 Post Trial 1. Upon completion of the trial, ensure that: a. All your work areas are left clean and tidy. b. All empty drums are sealed and stored safely. c. Pump area is clean and free from chemical spillage. d. All borrowed equipment is returned. 2. Reporting At the end of every field visit, a report should be submitted to the Production Supervisor giving a summary of the trial. This will form the basis of the meeting’s discussions. The report should include the following trial data: Production rates Demulsifier treat-rates BS&W throughout trial OIW throughout trial Timing of system, well changes This data is best represented in graphical form. Although it should also be written in tables. Always, wherever possible, include routine lab sample data collected by the operator. 2-39 – Chapter 2: Emulsions Always present a conclusion and a recommendation based upon your findings. Follow up with a full trial report upon returning from the field. 2.6 Application Methodology 2.6.1 General Statement of Application Put treatment as far away from the treating system as possible and as close to the problem as possible, and expose to heat as soon as possible. (One exception: a fast water dropper may reemulsify.) Emulsion breakers are normally injected into the produced fluid at: A well A problem well Headers Flowlines Before a transfer pump Before a heat exchanger Before a free-water knockout Before a treater and/or separator 2 .6. 2 Rule s of Thum b Stability of emulsions generally increases with age. Breaking emulsions as soon as possible after formation will eliminate or reduce the affects of aging. Oil with high viscosity has the ability to hold up more and larger water droplets than lower viscosity oil. Heat, diluent, and emulsion breakers can affect the viscosity of oil. The relative proportion of oil and water affects the stability of an emulsion. Typically lower water percentage in oil has a smaller chance for water droplets colliding, thus coalescing. Emulsifying agents such as solids, asphaltenes, bacteria, or acids are necessary to create emulsions. The elimination, alteration, or neutralization of these materials allows for resolution or prevention of emulsions. As system conditions dictate, PCC personnel should be prepared to recommend changes in the chemical injection points or rates. Chemical application depends on what avenues are available to you, what the system is, and what your Bottle Test tells you. 2-40 – Chapter 2: Emulsions 2.7 Troubleshooting The following is a general guide to resolving bad oil situations. When making treating changes, it is usually advisable to change only one parameter at a time. For example, do not increase the treater temperature and increase the demulsifier dosage at the same time, unless your experience tells you that this is the most successful course of action. 2.7.1 Bad Oil 2.7.1.1 Do Some Grindouts This procedure helps determine what has caused the bad oil. Do a complete system survey! Depending upon your field and system, the following are areas where grindouts need to be checked: 1. LACT Unit 2. Heater Treater a. Oil dump b. Water dump c. Sample cocks at various levels of the treater 3. FWKO a. Oil dump b. Water dump c. Sample cocks at various levels of the FWKO 4. Separators a. Incoming fluid b. Outgoing fluid 5. Wash Tanks or Gunbarrels a. Incoming fluid b. Outgoing fluid 2-41 – Chapter 2: Emulsions 6. Stock Tanks or Oil Holding Tanks a. Pipeline level b. Various levels in the tank When doing your grindouts: Use one sample for each location checked from above. Fill centrifuge tubes with a suitable hydrocarbon solvent to the 50% mark. (Do not use gasoline!) Fill the remainder of the centrifuge tubes with your samples of oil to the 100% mark. Do a quick spin first (about 15 to 30 seconds). This determines the amount of free water in your sample. Free water may indicate insufficient residence time or equipment malfunction. Centrifuge the sample according to API specifications. Slug the centrifuge tube with cut EC2003A and centrifuge again. This will determine any excess BS&W. Record all results. 2.7.1.2 Make Observations Regarding the Grindouts This procedure also helps determine what may have caused the bad oil. 1. If you observe High BS and this BS appears to be the same as the untreated BS in samples taken from producers in the field: Normally this indicates an undertreat. Actions to take are — − Check chemical pumps and chemical lines. Make sure that the chemical is actually being injected into the system at the dosage you intended. − Check treating equipment and vessels for proper operation. − Increase the chemical rate slightly. After a reasonable length of time, if high BS still exists, other possible solutions are: − Continue increasing chemical rate. − Change the emulsion breaker injection points; consult your system survey. − Slug the treating vessels and/or tanks with EC2003A. An average amount to use is one to five gallons per 100 barrels. 2-42 – Chapter 2: Emulsions − Again, make sure ALL production equipment is operating properly. − − − − Adjust flow of produced fluid through treating vessels. Adjust levels of produced fluid going through the treating vessels. Increase temperature on the treating vessels. Run an emulsion breaker Bottle Test. If possible, repeat the original Confirmation Test and compare the results. − Change demulsifier. 2. If you observe High BS and this BS is not like the untreated BS produced in the field: This indicates an undertreat of production coming from a new source or field operation. You must determine the source of this fluid. The possibilities could be: pit oil, tank bottoms, water treatment skimmings, workover fluid, new production from a different zone than the other producers in the field, deck drain fluid, etc. Actions to take are — − Identify the source of the incoming undertreated BS. − Follow Step 1, above. 3. If you observe High BS and your slugged grindout has more BS than the non-slugged grind out: Normally this indicates overtreatment. Actions to take are— − Check chemical pumps and decrease chemical rate. − Blend emulsified or untreated fluid with overtreated fluid. − Check treating vessels for proper operation. − Run an emulsion breaker Bottle Test on this overtreated fluid and recommend a different OFC emulsion breaker to be used. 2-43 – Chapter 2: Emulsions 4. If you observe the appearance of Solids (sand, iron sulfide, etc.): This also indicates chemical overtreat. This is normally caused by a well workover, a new producer has been put on line, pit oil circulation, slop oil circulation, skimmings from water treatment vessels, sumps, deck drains, etc. Actions to take are — − Determine where the solids are coming from, and isolate or divert to another treating system, if possible. − A complete shutdown of this incoming source of fluid may be recommended. − Review entire checklist from Step 1, above. − Slug production vessels and/or tanks with an OFC solids dispersant/water-wetting chemical. (To see a list of these chemicals, contact the product line manager or access OFC Vision on Lotus Notes.) 5. If you observe Low BS AND High Water or High Water Only: This can indicate — − A chemical overtreat. − Faulty dump valves on treating vessels. − A collapsed hay section in treating vessels. − A dirty treating vessel. Actions to take are — − Check the chemical pump rates and adjust accordingly. − If the present emulsion breaker is still overtreating, an emulsion breaker Bottle Test needs to be done, from which another OFC emulsion breaker should be recommended. − Check all dump valves on treating vessels to make sure they are operating properly. − Make sure treating vessel fluid levels are adjusted properly. Too high or low can cause free water to occur. − Check treating vessel temperatures. − Replace hay section. Soak hay with water and emulsion breaker first, before packing tightly into vessel’s hay section. − Clean out dirty treating vessels. 2-44 – Chapter 2: Emulsions 2.7.1.3 Summary of Root Causes All bad oil occurs because of a: Mechanical problem. Produced fluid problem. Chemical problem. Most frequently, bad oil occurs because of a mechanical problem or a produced fluid problem; seldom is bad oil a result of something chemical. The following is a summary of items to check when bad oil occurs. 1. Mechanical Problem Loss of heat Loss of vessel pressure Fluid levels are too high or too low Dump valves malfunctioning Vessel full of solids Vessel has a large floating BS pa Hay section has collapsed or needs to be cleaned out and replaced Electrical probes covered with scale New vessels in place, not lined out Loss of retention time, i.e.,vessel is being pushed with more produced fluid See also section on troubleshooting heater treater (below). 2. Produced Fluid Problem An increase or decrease of produced fluid (check well test data for each producer) Recycling tank bottoms, pit oil, skimmings, sumps, deck drains, etc. Well workovers: acid jobs, scale squeezes, solvent soaks, mud/acid flowbacks, oil well making solids, casing leak, paraffin scrapers, new well put on line, new zone completed, etc. An understanding of all the above is essential to becoming successful in resolving any bad oil situation. 2-45 – Chapter 2: Emulsions 2.7.2 Vertical and Horizontal Heater Treater s Bad oil or degradation in oil treating performance from a heater treater can have one or several of the root causes listed below: 1. Low temperature 2. Lack of demulsifier 3. Faulty dump valves 4. Chemelectric grids shorted out from high water cuts, paraffin, asphaltenes, iron sulfide, etc 5. Dirty treater 6. Severe pad/interface buildup 7. Overtreatment by demulsifier 8. Lack of retention time/treater crowded with too much incoming fluid for vessel to handle 9. Solids coating of grid in Chem-Electrics 10. Recycling too much or too often 11. Recycling of pit oil, skimmings, slop oil, etc. 12. Flowback of workover fluids 13. Increase in solids (sand, iron sulfide, etc.) 14. Loss of treater pressure 15. Loss of oil fired or gas fired burners 16. Loss of electricity for Chem-Electric and electrical problems 17. Low or high fluid levels in treater 18. Dirty produced water 19. Improper or ineffective demulsifier 20. Slug of biocide, corrosion inhibitor, surfactant, acid, etc., through vessel 21. Loss of gas to treater 22. New treater in place and not lined out 23. LACT unit rejecting to treat 24. Malfunction of production vessels upstream of treater Note: Not all situations mentioned above will necessarily yield bad oil. There may also be other conditions peculiar to your area or to a particular type of treater that will cause bad oil. Additionally, separators, flow-splitters, FWKOs, and gun barrels experience come of the above conditions. 2-46 – Chapter 2: Emulsions For the root causes listed above, here are some actions that may be successful in solving the problem. 1. Low Temperature Check burners to make sure they are lit. Check temperature gauges and replace if necessary. Check vessels upstream of the treater for heat loss/malfunction. Check burners for loss of fuel, pressure, etc. Check electrical power/Chem-Electric treater. Check recycling pumps for frequency of operation and source of recycled fluids. 2. Lack of Demulsifier Check chemical pumps. Check chemical lines. Check day tanks and chemical pots. 3. Faulty Dump Valves Check for proper operation. Have probe sensitivity checked by a technician. Check for solids accumulation at dump valve. Check for severe interface/BS pad buildup in treater. 4. Electrical Probes Scaled Off Clean probes or replace. Start a scale inhibition program for the temperatures seen in the treater. 5. Dirty Treater Check interface level; adjust if necessary. Check for faulty dump valves. Check incoming fluid to treater (workovers, recycling, etc.). Check for unusual pressure changes that cause rolling 2-47 – Chapter 2: Emulsions 6. Severe Pad/Interface Buildup Slug treater with slug/water wetter type emulsion breakers. Stop recycling. Check incoming fluid from field. Check chemical rates, chemical pumps, and chemical lines. Check for proper emulsion breaker. Run an emulsion breaker Bottle Test, observing effect of different products on the interface. Check for dirty treater. Check for workovers, etc., causing pad. Check for temperature loss. Check for slug of other chemicals (high volumes of corrosion inhibitors, etc.). Check the capacity of treating vessels; exceeding capacity reduces retention time. Program/product change may be required, e.g., diverting flow from treater. 7. Collapse of Hay Section Check for last hay replacement; suspect a problem if time since last replacement is longer than normal for the system or for your area. Check for increased fluid production from field (collapses due to excessive fluid). Check recycling frequency (collapses due to excessive recycling). Check for recent replacement of hay section (hay may not be packed properly, may pack itself with time). 8. Overtreat with Demulsifier — Bad Oil 9. Lack of Retention Time Check treater capacity rating and compare with production into treater. Split flow if possible. Check recycling frequency and adjust if necessary. 2-48 – Chapter 2: Emulsions 10. Recycling Check frequency and volume (excessive amounts will cause bad oil). Stop or adjust excessive recycling. Recycle fluids at a rate and volume that treater can handle. 11. Recycling Pit Oil, Slop Oil, etc. See 10, above. Check to see if recycled fluids need pre-treatment with a slop oil demulsifier. Isolate, or divert from normal production treater, if possible. 12. Flowback of Workover Fluids (same as mentioned in 11, above) 13. Solids (See 6, 10, and 11, above.) 14. Loss of Treater Pressure Check field gas source or alternate source of gas. Check for faulty gas regulator. Check for bad pop-off valve. Check for faulty dump valves. 15. Loss of Oil or Gas Fired Burners Check source of fuel. Check regulators. Check for water in the fuel. Check if burner needs to be replaced. Scale deposition on burner. 16. Loss of Electricity Check power source. Treat with slug/water wetter chemical. Float the pad/interface off. Check electrical probes for scale; recommend scale inhibitor if necessary. 2-49 – Chapter 2: Emulsions 17. Low/High Fluid Levels Check for faulty dump valves. Check for probe failure. See 8 through 14, above. Check vessels upstream of treater for malfunction. Check for bad oil. 18. Dirty Produced Water Check for faulty dump valves. Check for excessive recycling. Check for overtreat or undertreat. Check for excessive field gas, causing rolling of vessels. Check for malfunction of vessels upstream of treater. Check for faulty probes. Check inlet fluids (workovers, chemical slugs?). Check water clarification vessels for proper operation. 19. Emulsion Breaker Ineffective (See Troubleshooting Bad Oil.) 20. Chemical Slugs Check field operations for treatments performed, such as surface active biocide slugs, corrosion inhibitor slugs, paraffin treatments, etc. (See Troubleshooting Bad Oil.) 21. Loss of Treater Gas Check for faulty dump valves. Check source of gas. Check for wet gas. Check for malfunctioning gas regulator. 2-50 – Chapter 2: Emulsions 22. New Treater Check capacity and specifications of new treater. Check if inlet fluid rate is in excess of design capacity. 23. Lact Reject Check for mechanical or electrical failure. Check grindout. If good, call pipeline. If bad, see section on Troubleshooting Bad Oil. 24. Other Vessel Malfunction Determine vessel causing problem by analyzing grind outs. Take actions similar to those recommended in this section for treaters. See section on Troubleshooting Bad Oil. 2-51 – Chapter 2: Emulsions 2.8 Performance Targets/Measures The goal of the emulsion breaker (EB) program is to meet or exceed, at all times, the performance parameters set by the oil producer or refiner. In reality, many factors contribute to each area’s ability to meet this goal, but the emulsion breaker nevertheless plays a key role in the oil dehydration process. Measurable criteria for a successful EB program would be, but not limited to, the following: Chemical usage Operating and chemical costs associated with each facility should be reviewed monthly. Operating costs are normally monitored by the oil producer, where as chemical programs and usage should be monitored by the PCC Representative. Composition and quality of the interface fluids monitored in the treating vessels Interface composition and quality should be monitor weekly by checking levels in treating vessels. If vessels begin to show increased emulsion pad build up or poor oil, interface, or water quality, changes in the program may be necessary. Once a quality controlled program is established, and dependent on field operating changes, monitoring of vessels can be less frequent. Amount of waste oil generated There is cost associated with waste oil and water generated and re-treated. This should be monitored monthly. Flowline pressure Flowline pressure can frequently be attributed to emulsions. If this is found, check fluids for emulsion. If emulsions are found, quick water drop chemistries will normally work to reduce flowline pressures. However, if total dehydration is desired, further product screening may be necessary. Lowering flowline pressures may result in increased production due to fewer restrictions in the line. Daily production levels before and after initiation of an EB program will show how your EB program is working and can be reported as a Return On Investment (ROI). 2-52