Effect of Temperature on the Solubility of a Gas Lab Alka
advertisement

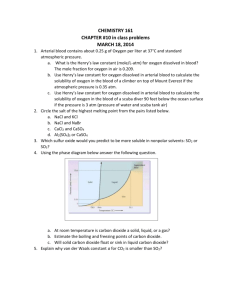
Effect of Temperature on the Solubility of a Gas Purpose: When Alka-Seltzer dissolves in water one of the products that is formed is carbon dioxide gas. The temperature of the water determines how much of this carbon dioxide actually becomes dissolved in the water. When carbon dioxide dissolves in water it forms carbonic acid, a weak acid. (H2O(l) + CO2(g) H2CO3(aq)). The more carbon dioxide that is dissolved the more acidic the solution becomes. If we can collect information about the resulting pH of a solution we can learn how temperature effects the solubility of a gas. We will get information about the pH of the solutions in this lab by two different means. First, we will use an indicator; a chemical that changes color with different pH’s. Secondly, we will use sodium hydroxide (a base) to neutralize the carbonic acid in the solution. The amount of NaOH it takes to neutralize each solution will indicate how acidic/how much carbon dioxide was dissolved in each solution. Directions: 1) Add 200 mL of TAP WATER (deionized water will be able to absorb CO2 from the atmosphere which will alter the results of the experiment) to each of three 250 mL beakers. Place one beaker in an ice bath, heat the second beaker on a hot plate, and allow the third beaker to stay at room temperature. Add into each of these beakers 3 mL of the bromthymol blue indicator. 2) Mass out two 1g samples of Alka-Seltzer in two weighing dishes. (It is okay if they don’t weigh exactly 1 g, but try to get the weights of the two samples as close to each other as possible.) 3) When the temperature of the water on the hot plate reaches between 75-80 °C remove it from the hot plate. At this time, also remove the cold beaker from the ice bath. Record the color of the bromthymol blue indicator at this point in the data table. 4) Simultaneously drop the two Alka-Seltzer samples into the hot and cold beakers. Observe and compare the evidence of physical and chemical changes in each beaker. 5) When the Alka-Seltzer tablets have fully dissolved (this means they have stopped fizzing), note the color of each solution in the data table. 6) Using a graduated cylinder, remove 25 mL of each sample from the beakers and move them into 3 appropriately labeled test tubes. 7) Using a pipette, add 1M NaOH dropwise into the cold water solution. Stir or swirl between drops to ensure thorough mixing. Count the number of drops of NaOH that must be added to match the color of the room temperature control solution. Record this number in the data table. 8) Repeat step 7, except use the hot water solution. Data: Initial Color of Bromthymol Blue before Alka-Seltzer was added Color of Bromthymol Blue after Alka-Seltzer was added Number of drops of 1M NaOH Cold Solution Room Temperature Solution Hot Solution ** The more acidic the solution, the more yellow the bromthymol blue; the more basic the solution, the more blue the bromthymol blue.** Analyzing the Data: 1) Which solution, cold or hot, was the more acidic? Explain how you know this. 2) Which of these solutions then had the most CO2 dissolved in solution? Explain how you know this. 3) Explain how did the number of drops of 1M NaOH needed to neutralize the cold and hot solutions support your answers in questions 1 and 2. 4) Suppose you took the cold solution after all the Alka-seltzer had dissolved and heated it on the hot plate. Explain what would happen to the color of the bromthymol blue and why it would change to that color. 5) Explain how the solubility curve for the dissolving of CO2 in water would be different than the solubility curve for dissolving KNO3 in water. 6) Think about what you know about the molecular differences between a gas and a solid. Explain how this could help explain the difference in the solubility of a gas and a solid at the same temperature. Application Questions: 7) A cold soda when opened is very “fizzy” with carbonation. Explain why the soda becomes “flat” as it reaches room temperature. 8) Power plants are usually located near lakes or streams because they use large amounts of water in the process of generating electricity. The energy from burning coal or natural gas turns water into steam and then this steam is used to turn a turbine, which generates the electricity. Thermal pollution of a lake or stream occurs when a power plant fails to cool the water used to generate the steam before putting it back into the lake or stream. The water around these plants often times does not support many aquatic species. Why? (And don’t say because the water is too warm!! Think about what you learned in this lab.)