PCPNDT Act - Guidelines
advertisement

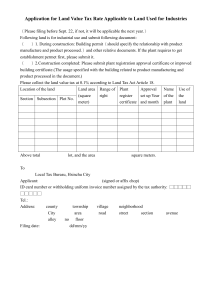
PCPNDT ACT - GUIDELINES 1. Register all the places wherever USG machine/any imaging machine like Colour Doppler/2D/3D/4D /CT/MRI/Opthal/Lithotripsy with USG machine is used, in medical/ surgical Nursing Home/ICCU wherever USG machine is used every centre needs to be registered under PCPNDT ACT. 2. If portable machine is used then as per High Court order it should be used in only one centre. It can not be shifted out of the centre as potability is banned to avoid misuse of machine. 3. All IVF centres to be registered as Genetic Clinic, and Genetic Labs and Genetic Counselling centres by payment of fees as combined centres of Rs. 35,000/-. Application should be forwarded to Advisory Committee for recommendation by every MOH. It shouldn’t be registered at their own level, if any IVF centre is still not registered necessary action under PCPNDT Act to be initiated by concerned MOH as appropriate authority as per instruction of Government. 4.Total number of machines, company name & make of every USG Serial no. of machine should be noted in PCPNDT registration certificate by MOH of ward in original PCPNDT Certificate. 5.All radiologist /sonologist / Doctors using machines, their names to be entered in the PCPNDT certificate or on the separate sheet to be displayed along with PCPNDT registration certificate. 6.In case of Genetic Lab-Name of Geneticist/Embryologist also to be added in the PCPNDT Registration Certificate. No embryologist or Geneticist or techinician should be allowed to work in the Genetic Laboratory till the name of Embrologist/genetist/qualified Lab technician is registered in PCPNDT certificate. 7.No doctor other than registered in that particular centre should use that machine. Locum qualified doctor may use that machine after notifying to Appropriate Authority and their names being added in the registration certificate. 8.Display Registration Certificate in Original in Waiting Area and USG room/Genetic Clinic/Genetic Lab. 9.Display on notice board the message in English and local language that "Sex Selection and Detection is not done in this Centre" and is punishable under the act. This should be displayed in Waiting Area and USG Room/Clinic/Genetic Lab. etc. 10. Any complaint to be registered on www.amchimulgi.com. Helpline No. 18002334475. All the centres to display this massage for public. 11.Various forms under PCPNDT Acti.”A”-Fom- Application form to be submitted in duplicate certificate. ii- “B”-Form- Registration certificate iii-“C”form- Rejection certificate if application is not recommended to be given within 90 days. iv."D" Form - To be maintained by Genetic Counseling Centres only. v."E" Form-To be maintained by Genetic Laboratories and copy of E form to be submitted to MOH monthly along with monthly report. vi. "F" Form to be maintained by USG Centre / Genetic Clinic as per PCPNDT Act for all pregnant patients only. a. All column mentioned in “F” form should be filled up, no Column should be added/ deleted. b. Previous Obst. History of patients with number of children with sex of each child should be mentioned in “F” Form. c. “F” Form to be filled up for all pregnant women in Duplicate and all forms to be down loded on mahaonline.gov.in site and for every case print out and hard copy to be maintained by centre. d. Name of referral doctor, indication, results of USG must be mentioned whichever column is not required must be filled up as not applicable /No. e. Monthly report to be printed and attested by centre and filed with centre & copy to be e mailed to M.O.H. and Special Officer, F.W. & M.C.H. f. “F” form should be signed by doctor conducting sonography & not by the owner of the centre. g. Declaration of the patient and doctor should be signed before doing the Sonography and after explaining the patient in her own language as per the Act. vii.G Form to be filled by Genetic Clinic as a consent of woman for doing invasive procedure. viii. "H" Form to be maintained & updated time to time by Appropriate Authorities & to be informed to Special Officer, F.W. & M.C.H. ix. Common register to be maintained by all centres for all the cases in the format for all PCPNDT Centres (for male/female cases). Sr.No. Name of the person Address & Name of spouse Date of Undergoing PNDT Contract No. of father examination procedure 1 2 3 4 5 Monthly report should be sent in time by 1st of month by all PNDT registered Centres to 12. Appropriate Authority and on email id sofwunit@gmail.com by 3rd of month by A.A. to Special Officer, F.W. & M.C.H./M.I.S. 13. All records of all the patients to be maintained for minimum 2 years or if any legal case against the centre is pending then records to be maintained till the same case is disposed off. 14. Any changes in centre - like machine / Doctor should be intimated to A. A. within 30 days. Accordingly He/She should do changes in registration certificate immediately and inform Special Officer, F.W. & M.C.H. in writing and also does the changes in "H" Register. 15. As per PCPNDT Act for renewal of Registration, it is mandatory to apply one month in advance to A.A. Otherwise centre will be considered as un-registered. Machines can be sealed even 2-3 months in advance. If application is not made one month in advance – machine can be sealed by following due procedure. 16. M.O.H.(Appropriate Authority) should remind to concerned centre regarding date for renewal at least 2 months in advance to the centre. 17. Application form "A" to be submitted in duplicate along with documents of machine and undertaking duly notarized stating Sex Selection/ & Sex Detection is not done in Centre and Centre will display the message in English & Local language that Sex Selection & Detection will not be done at the Centre which is punishable under the act. 18.Advertisement of any sort is banned even on internet, or coding form – Punishable under the act - Rs.1 Lac fine with 5 years imprisonment. 19. Online submission of “F” Form to be done any queries regarding Mahaonline to be enquired on email mahaonline.gov.in and Help line No. 1. pnd07@gmail.com Contract Details: 020-26058476/Ext.124 2.punam.mahajan@mahaonline.gov.in Contract Details:-022-67077017 20.All machines are to be registered ane every machine is given machine registration number and certificate and the MRC No. will be embossed or sticker will be fixed on every machine. 21.M.O.H. to inspect all centres (PCPNDT/MTP) atleast once in 3 months. 22.All court cases filed-are downloaded online on www.mhpcpndtcourtcases.org.in /login.aspx day to day progress and case details to be down loaded within 48 hours of court proceedings by Appropriate Authority. 21.Every M.O.H. to keep inspection report of every centre and “H” Form to be updated immediately after any changes are approved by Committee/Appropriate level and to be informed to Special Officer, F.W. & M.C.H. 23.If any centre is not functioning or machine is not working no PCPNDT Centre registration to be cancelled without proper proceeding of USG Machine as no machine to be kept unregistered in any place/USG Machine has to be properly registered first in another place/voluntarily sealed/destroyed/or handed over same company. Company letter to be received and then only existing machine to be cancelled by Appropriate Authority after getting approval of Executive Health Officer. 24.No changes/No temporary permissions to be given at Appropriate Authority level for use of machine/cancellation/registration or temporary permissions. Sd/Executive Health Officer