Advanced Reactions Unit Homework Assignments
advertisement

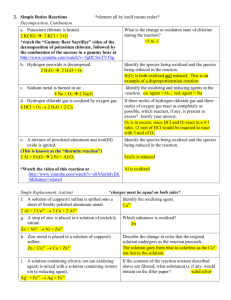
Advanced Reactions Unit Homework Answers Oxidation Number Practice For each of the following list the oxidation numbers for each element: (You must show work) 1. S2O32S= +2, O= -2 5. AlCl3 Al= +3, Cl= -1 2. N2H4 N= -2, H= +1 6. KClO3 K= +1, Cl = +5, O= -2 3. HIO3 H= +1, O= -2, I= +5 7. PO43P= +5, O= -2 4. NOF O= -2, F= -1, N= +3 8. H2O2 H= +1, O= -1 (peroxide) Oxidation-Reduction Identification Practice Identify the element oxidized, the element reduced, the species that is the oxidizing agent, and the species that is the reducing agent. (You must show oxidation numbers for each element.) 1. N2H4+ NaBrO3N2+ NaBr 4. As + ClO3- H3AsO3 + HClO Br reduced, NaBrO3 oxidizing agent Cl reduced, ClO3- oxidizing agent N oxidized, N2H4 reducing agent As oxidized, As reducing agent 2. SO32-+ MnO4-Mn2++ SO42Mn reduced, MnO4- oxidizing agent S oxidized, SO32- reducing agent 5. SO42- + Br - Br2 + SO2 S reduced, SO42- oxidizing agent Br oxidized, Br- reducing agent 3. H2O2+ OCl-O2+ Cl2 Cl reduced, OCl- oxidizing agent O oxidized, H2O2 reducing agent LeChatelier’s Principle Practice Consider the following equilibrium reaction: N2(g) + 3H2(g) 2NH3(g) exothermic For each of the following scenarios, specify the direction in which the equilibrium will shift: 1. NH3 is removed – shifts R 2. The pressure on the reaction is decreased – shifts L 3. N2 is added – shifts R 4. The mixture is heated – shifts L Acid Base Reaction Practice Write out the following reactions using appropriate symbols and balance them. 1. 2HCl(aq) + Ba(OH)2(aq) BaCl2(aq) + 2H2O(ℓ) 2. HNO3(aq) + RbOH(aq) RbNO3(aq) + H2O(ℓ) 3. 2HC2H3O2(aq) + Ca(OH)2(s) Ca(C2H3O2)2(aq) + 2H2O(ℓ) 4. H2SO4(aq) + 2NaOH(s) Na2SO4(aq) + 2H2O(ℓ) 5. 3H2SO4(aq) + 2Fe(OH)3(s) Fe2(SO4)3(aq) + 6H2O(ℓ) 6. HNO3(aq) + NaC2H3O2(s) HC2H3O2(aq) + NaNO3(aq) pH Calculations Practice You must show the equations used and the substitutions in order to receive credit for this. On tonight’s homework, write Go Wolfpack ON THE BACK (even if you are a Carolina fan)– please do not tell your friends! What is the pH of a solution with [H3O+] of 1.50x10-3M? 2.82 What is the pH of a solution with [OH-] of 0.0325M? 12.5 What is the pOH of a solution with [OH-] of 0.00025M? 3.6 What is the pOH of a solution with [H3O+] of 5.0x10-9M? 5.7 What is the pH of a 0.15M hydrochloric acid solution? 0.82 A saturated calcium hydroxide solution has a concentration of 0.0118M. What is the pH of this solution? 12.4 Rainwater is naturally acidic because it tends to be nearly saturated with carbon dioxide. A saturated solution of carbon dioxide under typical atmospheric conditions has a pH of 5.65. What is the [H3O+] in this solution? 2.24x10-6M 8. The pH of Diet Dr. Pepper is around 2.4. What is the [OH-] in Diet Dr. Pepper? 2.5x10-12M 1. 2. 3. 4. 5. 6. 7.