Coverage Update, BCG, ADT vaccine shortage
advertisement

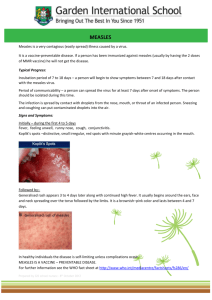
1-3 The Terrace P.O Box 5013 Wellington Date: 3 October 2014 Pages: 1 of 2 To: General Practitioners, Practice Nurses, Practice Managers, Health Professionals From: Rayoni Keith, Manager, Immunisation Subject: Coverage Update, BCG, ADT vaccine shortage, Synflorix to Prevenar 13, Infanrix-IPV packaging, Meningitec Recall, Measles, Ebola Coverage update from Immunisation Champion Dr Pat Tuohy The provisional coverage has increased to over 92 percent. From October, the Ministry will be celebrating those practices that have reached 100% infant immunisation coverage during that calendar month. We appreciate the effort that is put in, especially to reach those last couple of children and we want to thank those practices for their dedication. The difference between 92 and 95 percent is only a small number of children. 229 children around the country will need to complete their 5 month immunisation during October, before they turn 8 months old, in order for 95 percent of this age group to be protected. One of these children could be in your practice – if you are not sure, please contact your NIR administrator or immunisation co-ordinator. BCG BCG vaccine has now been licensed by Medsafe and is once again an approved medicine in New Zealand. All BCG vaccine clinics should have now recommenced. ADT Booster (Td) vaccine shortage ADT Booster vaccine (Td), which is offered at ages 45 and 65 years and for tetanus prone wounds, is back in stock at ProPharma. If you have been using Tdap (Boostrix) in the short term as a replacement for Td (ADT Booster), please return to using Td as Tdap is not funded for 45 and 65 year olds and tetanus prone wounds. Synflorix to Prevenar 13 Please ensure that all Synflorix stock is used up before you begin to administer Prevenar 13 to patients and that the correct batch number is entered into your PMS. Infanrix-IPV packaging GSK have advised that they will shortly be replacing the green packaging for Infanrix-IPV with orange packaging, in order to avoid confusion with Synflorix. For a period of time, there will be a combination of both orange and green packs in the market. For further information or if you have any concerns, please contact GSK Medical Information on 0800 808 500. Meningitec recall All unexpired doses of Meningitec (Meningitec meningococcal serogroup C conjugate vaccine suspension for injection, single dose syringe) are being recalled worldwide, including New Zealand. A review of batches manufactured since October 2012 found a small number of syringes had been contaminated with iron oxide (rust) and oxidised stainless steel, both of which originated from manufacturing equipment. The risk that someone has received a contaminated dose of vaccine is very low. Only a small number of doses per batch were found to be contaminated, and the product manufacturer indicates that there have been no reported adverse events associated with this issue worldwide. Healthcare Logistics will be sending recall faxes to practices known to have received stock since February this year, with those having received stock earlier than this being contacted over the next few days. The Ministry recommends the following alternative conjugate meningococcal vaccines: Neisvac-C from Baxter and Menactra from Sanofi-aventis. Measles At least fifteen children in Syria died in mid-September shortly after the administration of a measles vaccine. While the WHO has suspended the vaccination programme in Syria pending an investigation into the deaths, the Ministry has received assurance from New Zealand’s supplier of MMR vaccine that the affected vaccine in Syria is not the same as that used in New Zealand. We do not consider that there is any increased risk to patients in New Zealand. Early reports suggest that the vaccine in Syria was reconstituted with a muscle relaxant that was stored with the vaccines, rather than the correct diluent. This tragic event highlights the importance of proper handling and storage of medicines. The Ministry recently declared the recent measles outbreak over after two incubation periods without a case being reported. Until immunisation rates among older children, teenagers and young adults increases, we expect such outbreaks will continue to occur in the future, here and overseas. We also expect further isolated measles cases to be reported. On 30 September the Ministry was informed of a measles case in Wellington. The Ministry is considering plans to achieve measles elimination in New Zealand. We ask that general practices continue to offer MMR to anyone born after 1 January 1969 who has not had two doses of measles vaccine, and record the vaccination on the NIR. Ebola A man who has fallen ill after travelling from Liberia to the United States has been confirmed as having Ebola. The Ministry of Health assessment is that the risk to New Zealand from Ebola remains low. Border screening is already in place for individuals arriving from West African countries, such as Liberia, affected by the Ebola outbreak. These controls are similar to those in place in comparable countries. Since screening was introduced early in August there have been 47 people screened - none have had symptoms. An expert advisory group has been established so the Ministry can continue to check its precautions are appropriate. The Ministry of Health is closely monitoring the situation in the United States, and will continue to monitor the advice from, and actions being taken by, the World Health Organization and other countries in relation to the Ebola outbreak in West Africa. For more information and latest updates, see www.health.govt.nz/ebola. Any queries should be directed to Ebolareadiness@moh.govt.nz. If you have any queries about anything in this update, please email immunisation@moh.govt.nz.