Homework #9 - University of Idaho
advertisement

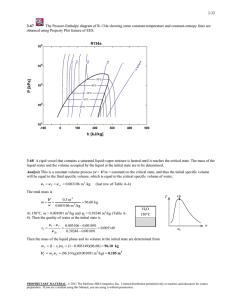
ME 322 – Mechanical Engineering Thermodynamics Spring 2016 Homework #9 MULTI-CHOICE (circle all correct answers; supply supporting reasoning if in doubt) 9-1 What are valid units of the universal gas constant, Ru? a) Btu/(lbm-R) b) kJ/(kg-K) c) kJ/(kgmol-K) d) ft-lbm/(lbmol-R) 9-2 Which of the following quantities have MLt units of L2/t2? a) kinetic energy b) specific heat transfer c) specific work transfer d) potential energy 9-3 What is the thermodynamic name for a process where enthalpy is constant? a) adiabatic b) aergonic c) isentropic d) isenthalpic 9-4 Which are valid expressions for work of an ideal gas during an isobaric process? a) p (V2-V1) b) V (p2-p1) c) mR(T2-T1)/(1-n) d) ʃp dV PROBLEMS Solve 3.48 from the course textbook using EES for a range of ending pressures extending from 2.00 MPa to atmospheric pressure. Plot the resulting steam quality as a function of ending pressure. Use EES to create a p-H (pressure-enthalpy) diagram for water that focuses on saturation behavior up to the critical point. Illustrate/explain how you can use this chart to validate your solution to 3.48. Solve 3.55 from the Balmer text using EES. Verify your answers against those from the thermodynamic tables supplement. Discuss issues you encountered with each method. Note: On the course website you will find examples in the required homework format for EES Solutions. Please look these over as you prepare your solution. Homework is due at the beginning of the next class period, unless specified otherwise on the course website. Homework not handed in at the beginning of the class period is late and will not be graded.