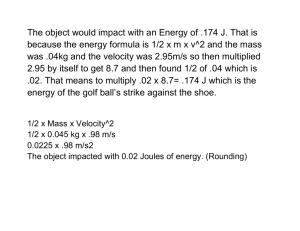Phase velocity - Book Spar | Website for students | VTU notes
advertisement

www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
UNIT-1
MODERN PHYSICS
Introduction to blackbody radiation spectrum:
A body which absorbs all radiation that is incident on it is called a perfect blackbody. When radiation
allowed to fall on such a body, it is neither reflected nor transmitted. Such a black body after absorbing the
incident radiation gets heated and starts emitting radiation of all possible wavelengths. In practice, perfect
blackbody does not exist and we can have objects that are only close to a blackbody. A blackbody, on heating,
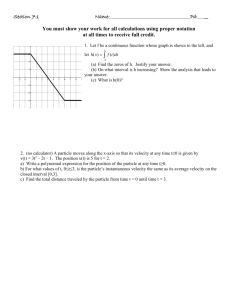
can emit all radiations it has absorbed and is called blackbody radiation. Figure shows the energy distribution
curves in which energy density E (energy emitted by the blackbody per unit area of the surface) is plotted as a
function of wavelength at different temperatures of the blackbody.
The important points that can be noted down from these curves are
1. All curves shows a peak suggesting that the emitted intensity is maximum at a particular wavelength.
2. An increase in temperature results in an increase in the energy emitted.
3 .As the temperature increases, the peak shifts to lower wavelength.
Laws of blackbody radiation:
1. Stefan-Boltzmann law
It states that the total energy density E0 of radiation emitted from a blackbody is directly proportional to
the fourth power of its absolute temperature T.
i.e., E0 T4
Or E0 = T4
where is a constant called Stefan’s constant with numerical value equal to 5.67×10-8 Wm-2K-4. This law
agrees well with the experimental results.
2. Wien’s displacement law
It states that the wavelength m corresponding to the maximum emissive energy decreases with
increasing temperature.
𝟏
i.e., m T
Or m T = b,
where b is called the Wien’s constant and is equal to 2.9×10-3 mK.
3. Wien’s law
Using laws of thermodynamics and classical concepts, Wien developed an expression for energy density
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 1
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
as,
𝐔(𝛌)𝒅𝝀 =
𝐂𝟏 𝟏
𝒅𝝀
𝐂𝟐
𝛌𝟓 𝛌𝐓
𝐞
where C1 and C2 are constants.
This law holds good for smaller values of wavelength.
4. Rayleigh-Jeans law
Rayleigh derived an expression for the energy density of radiation based on classical theory which is
given by,
𝐔(𝛌)𝒅𝝀 =
𝟖𝛑𝐤𝐓
𝛌𝟒
𝒅𝝀
where k is called Boltzmann’s constant and its value is 1.381 x 10-23JK-1
This law holds good only for large values of wavelength.
As per the Rayleigh-Jeans law, the radiant energy increases with decreasing wavelength and a
blackbody must radiate all the energy at very short wavelength. But in actual practice, it doesn’t happen so. The
failure of Rayleigh-Jeans law to explain the aspect of very little emission of radiation beyond the violet region
towards the lower wavelength side of the spectrum is referred as ultraviolet catastrophe.
Both Wien’s law and Rayleigh-Jeans law indicates failure of classical theory in explaining blackbody radiation.
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 2
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
Planck’s radiation law:
Max Planck proposed a law based on quantum theory. According to this, atoms or molecules absorb or
emit radiation in quanta or small energy packets called photons. If ‘’ is the frequency of photons, then its
energy can be explained as E=h where h=6.63x10-34Js is called Planck’s constant. Applying quantum theory,
Planck obtained an expression for energy density of blackbody radiation as,
𝐔(𝛌)𝒅𝝀 =
𝟖𝛑𝐡𝐜
𝟏
𝛌𝟓
𝐡𝐜
𝐞𝛌𝐤𝐓 −𝟏
𝒅𝝀 ----------------- (1)
This law agrees well with the experimental observation of blackbody radiation and is valid for all wavelengths.
1. For shorter wavelengths:
𝐡𝐜
i.e., when λ is small, 𝐞𝛌𝐤𝐓 is very large
𝐡𝐜
𝐡𝐜
𝐡𝐜
Or i.e., 𝐞𝛌𝐤𝐓 >>1 hence (𝐞𝛌𝐤𝐓 − 𝟏) ≈ 𝐞𝛌𝐤𝐓
Substituting this in Planck’s radiation law i.e., in eq(1) then,
𝟖𝛑𝐡𝐜 𝟏
𝐔(𝛌)𝒅𝝀 =
𝒅𝝀
𝐡𝐜
𝛌𝟓 𝛌𝐤𝐓
𝐞
𝐂𝟏 𝟏
i.e., 𝐔(𝛌)𝒅𝝀 = 𝟓 𝐂𝟐 𝒅𝝀
𝛌
𝐞𝛌𝐓
hc
Where C1=8hc and C2= k
Hence at smaller wavelengths, Planck’s radiation law reduces to Wien’s law.
2. For longer wavelengths:
𝐡𝐜
i.e., when λ is large, 𝐞𝛌𝐤𝐓 is very small
Expanding the power series , we have
𝐞
Since
𝐡𝐜
𝛌𝐤𝐓
𝐡𝐜
𝛌𝐤𝐓
=𝟏+
𝒉𝒄
𝝀𝒌𝑻
𝟏!
+
𝒉𝒄 𝟐
)
𝝀𝒌𝑻
(
𝟐!
+−−−−−−−
is very small, its higher power terms can be neglected
Then the above expression becomes
𝐡𝐜
𝐞𝛌𝐤𝐓 = 𝟏 +
hc
λkT
𝐡𝐜
or 𝐞𝛌𝐤𝐓 – 𝟏 =
hc
λkT
Substituting this in Planck’s radiation law i.e., in eq(1),
𝟖𝛑𝐡𝐜 𝟏
𝐔(𝛌)𝒅𝝀 =
𝒅𝝀
𝛌𝟓 𝐡𝐜
𝛌𝐤𝐓
i.e., 𝐔(𝛌)𝒅𝝀 =
𝟖𝛑𝐤𝐓
𝛌𝟒
Hence at longer wavelengths, Planck’s radiation law reduces to Rayleigh-Jeans law.
Photoelectric effect:
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 3
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
Emission of electrons from a metal surface when light of suitable energy falls on it is called
photoelectric effect. The experimental setup for observing photoelectric effect consists of a pair of metal plate
electrodes in an evacuated discharge tube connected to a voltage source as shown:
When light of suitable energy is incident on the cathode, electrons are emitted and a current flows across the
discharge tube. Some special features of photoelectric emission are:
1. It is an instantaneous process-there is no time interval between the incidence of light and the emission of
photoelectrons.
2. There is a minimum frequency for the incident light called threshold frequency, below which no photoelectric
emission occurs. This depends upon the nature of the material of the emitter surface. The energy
corresponding to the threshold frequency, called the work function is the minimum energy required to release
an electron from the emitter surface.
3. For a given frequency of the incident light, photocurrent is directly proportional to the intensity of the
incident light.
4. The photoelectron emission can be stopped by applying a reverse voltage to the phototube. ie., by making the
emitter electrode positive and the collector negative. The negative collector potential required to stop the
photoelectron emission is called the stopping potential.
Einstein’s Theory:
Photoelectric effect can be explained on the basis of quantum theory of light. When the energy equal to
work function of the metal is incident on the metal surface, the incident photon liberates electrons from their
bound state. When the incident photon carries energy in excess of the work function, the extra energy appears as
the kinetic energy of the emitted electron. When the intensity of light increases, the number of photoelectrons
emitted increases but their kinetic energy remain unaltered. When a photon of frequency ‘’ is incident on a
metal surface of work function ‘’, then,
𝟏
h = + ( 𝟐 mv2)max
1
where ( 2 mv2)max is the kinetic energy of the emitted photoelectrons.
This is known as Einstein’s photoelectric equation.
Since = h0, it can also be written as,
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 4
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
1
( 2 mv2)max = h - = h(-0)
If V0 is the stopping potential corresponding to the incident photon frequency , then,
1
( 2 mv2)max = h -
1
𝟏
( 2 mv2)max = h(-0) or ( 𝟐 mv2)max = eV0
Compton Effect:
When X-rays are scattered by a solid medium, in addition to the scattered X-rays of same frequency,
there exist some scattered X-rays of a slightly lower frequency (higher wavelength). Compton observed this
phenomenon and is called Compton Effect.
Compton Effect can be explained on the basis of the quantum theory and laws of conservation of energy and
momentum. Consider an x-ray photon of energy h incident on an electron at rest.
After the interaction, the X-ray photon gets scattered at an angle with its energy changed to h’ and
the electron which was initially at rest recoils at an angle . It can be shown that the increase in wavelength is
given by
h
∆λ=
(1- cos θ)
m0 C
h
When = 900, = m
0
where m0 is the rest mass of the electron.
= 0.0242Å. This constant value is called Compton wavelength.
C
Wave particle dualism:
The Photoelectric Effect and Compton Effect conclusively established the particle behavior of light. The
phenomena of interference, diffraction and polarization give exclusive evidence for the wave behavior of light.
Hence we have to conclude that light behaves as an advancing wave in some phenomena and it behaves as a
flux of particles in some other phenomena. Therefore we say that light exhibits wave-particle duality.
De-Broglie’s hypothesis:
De-Broglie extended the wave particle dualism of light to the material particles. This is known as deBroglie hypothesis. According to this hypothesis, material particles in motion possess a wave character. The
waves associated with material particles are called matter waves or de-Broglie waves.
According to Planck’s theory of radiation,
E = h
--------- (1)
where is the frequency associated with the radiation.
According to Einstein’s mass-energy relation,
E = mc2
--------- (2)
where m is the mass of the photon and c is the velocity of light
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 5
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
Combining (1) and (2),
hc
𝑐
i.e., h = mc2 =>
= mc 2
(since 𝜐 = )
λ
h
𝜆
= mc
λ
Therefore momentum associated with the particle is given by p = mc,
Or, =
𝒉
where is called de-Broglie wavelength.
𝒑
De-Broglie wavelength associated with the accelerated electron:
A beam of high energy electrons can be obtained by accelerating them in an electric field. Consider an
electron starting from rest when accelerated with a potential difference V, the kinetic energy (E) acquired by the
electron is given by,
1
E= mv2 and also E= eV
2
Thus,
Or
1
mv 2
2
m2 v 2
2m
= eV
= eV
p2
i.e.,
=eV =E
2m
where ‘v’ is the velocity of the electron, ‘m’ its mass and ‘p’ the momentum.
Now the momentum may be expressed as,
p = √2mE = √2meV
Hence the de-Broglie wavelength λ=
h
p
=
h
√2mE
=
h
√2meV
To test de-Broglie hypothesis, Heisenberg and Schrödinger formulated theories whereas G.P.Thomson,
Davisson and Germer conducted experiments.
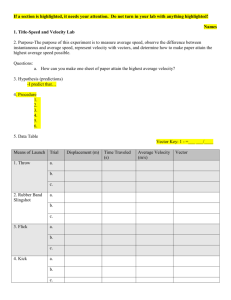
Davisson-Germer experiment:
The electron diffraction experimental setup used by Davisson and Germer to verify de-Broglie’s
hypothesis is as shown:
The filament F is heated to produce electrons via thermionic emission. These electrons are passed
through a narrow aperture forming a fine beam of accelerated electrons. The electron beam was then made to
incident on a single crystalline sample of Nickel. The electrons scattered at different angles were counted using
a detector. The experiment was repeated by recording the scattered electron intensities at various positions of
the detector.
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 6
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
A sharp maximum occurred in electron density at an angle (Φ) of 500 with the incident beam for an
accelerating potential of 54 V. The angle of incidence corresponding to this is 250 and from figure, glancing
angle θ (angle of diffraction) is 650. From X-ray diffraction experiment, spacing of the planes responsible for
diffraction was found to be 0.091 nm.
Assuming first order diffraction, Bragg’s law can be written as,
= 2d sin = 2×0.091×10-9×sin65 = 0.165 nm.
By de-Broglie relation,
𝛌=
𝐡
√𝟐𝐦𝐞𝐕
=
𝟔.𝟔𝟐𝟔 × 𝟏𝟎−𝟑𝟒
√(𝟐×𝟗.𝟏×𝟏𝟎−𝟑𝟏 ×𝟏.𝟔×𝟏𝟎−𝟏𝟗 ×𝟓𝟒)
= 0.167nm
Thus Davisson and Germer experiment directly verifies the de-Broglie hypothesis.
Characteristic properties of matter waves:
1. Matter waves are associated with moving particle.
2. Wavelength of matter waves is inversely proportional to the velocity with which the particle is moving
h
( =mv). Hence a particle at rest has an infinite wavelength.
3. Wavelength of matter waves is inversely proportional to the mass of the particle. Hence wavelike behavior
of heavier bodies is not very evident whereas wave nature of subatomic particles could be observed
experimentally.
4. Wave function is used to define a matter wave which is related to the probability of finding a particle at any
place at any instant.
5. Matter waves are represented by a wave packet made up of a group of waves of slightly differing
wavelengths. Hence we talk of group velocity of matter waves rather than the phase velocity (velocity of a
single wave). The group velocity can be shown to be equal to the particle velocity.
Phase velocity:
General expression for a wave is Y = A cos (t-kx)
where Y = Displacement at any instant t, A = Amplitude of vibration, = 2 is the angular frequency and
2𝜋
k = 𝜆 is the wave vector or wave number.
Phase velocity or wave velocity of a wave is the velocity of the wave when phase is constant.
i.e., t-kx = constant
or, kx = t + constant
ωt
or, x = k + constant
Hence Phase velocity vp =
𝐝𝐱
𝐝𝐭
=
𝛚
𝐤
Group velocity:
The de-Broglie waves are represented by a wave packet and hence we have ‘group velocity’ associated
with them. Group velocity is the velocity with which the wave packet travels.
Consider two waves having same amplitude but having slightly different frequency and wave number
represented by the equations
Y1 = A cos (ωt-kx)
Y2 = A cos [(ω+ Δω) t – (k+ Δk) x]
The resultant displacement due to the superposition of the above two waves is,
Y = Y1 + Y2
= A cos (ωt-kx) + A cos [(ω+ Δω) t – (k+ Δk)x]
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 7
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
A B
A B
Since, cos A + cos B = 2cos (
) cos (
),
2
2
2
2k k
k
Y = 2A cos {(
)t–(
) x} cos {(
)t (
) x}
2
2
2
2
As the difference in frequency of the two waves is very small, we can assume,
2+Δω 2 and 2k+Δk 2k
k
Y = 2Acos {(
)t (
) x} cos (ωt-kx)
2
2
The velocity of the resultant wave (group velocity) is given by the speed with which a reference point, say the
maximum amplitude point, moves. Taking the amplitude of the resultant wave as constant, we have,
k
2Acos {(
)t (
) x} = constant
2
2
k
or, (
)t (
) x = constant
2
2
or, x =
∆𝜔𝑡
∆𝑘
+ constant
𝒅𝒙
∆𝝎
Group velocity vg = 𝒅𝒕 = ∆𝒌
When and k are very small,
𝐝𝛚
vg =
𝐝𝐤
Relation between group velocity and phase velocity:
𝜔
Phase velocity, vp = 𝑘
where ω is the angular frequency of the wave
= vp k ----- (1)
𝑑𝜔
Group velocity, vg = 𝑑𝑘
vg =
dvp k
(From eq (1))
dk
dk
dvp
2𝜋
dk
dvp
= vp dk + k
= vp + ( 𝜆 )
1
1
d( )
𝜆
dvp dλ
1
1
d( ) dλ
𝜆
dvp dλ
= vp + ( 𝜆 )
= vp + ( 𝜆 )
= vp + ( 𝜆 )
But
1
λ
d( )
dλ
=
where the propagation constant (or wave number) k =
2𝜋
d( )
𝜆
dvp
2𝜋
𝜆
1
dλ d(1)
𝜆
−1
λ2
1 dvp
Therefore vg= vp+ (𝜆)
dλ
λ2
( −1)
Thus vg= vp- 𝝀
𝐝𝐯𝐩
𝐝𝛌
Relation between group velocity and particle velocity:
E
Energy of a photon E = hν or ν = h
------ (1)
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 8
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
(2E)
We know the angular frequency of the wave ω = 2ν or ω = h
2
dω = ( h ) dE ------(2)
2
Further, k =
=
λ
2p
since =
h
h
p
2
dk = ( h ) dp
------(3)
Substituting the value of d and dk from equations (2) and (3) in the expression for group velocity,
dω
dE
vg = dk = dp ------ (4)
If a particle of mass m is moving with a velocity vparticle, its energy is given by,
𝑝2
1
E = 2 mv2particle = 2𝑚 -------(5)
Substituting this in equation (4),
dE
vg = dp =
𝑝2
2𝑚
d
p
dp
=m
mVparticle
=
= vparticle
m
Hence vg = vparticle
Relation between velocity of light, group velocity and phase velocity:
Phase velocity, vp =
𝜔
𝑘
where k is the propagation constant or wave number
We know the angular frequency of the wave ω = 2ν or ω =
Further, Wave number k =
Thus
vp =
=
2
=
λ
𝜔
2p
since =
h
h
p
(2E)
h
where p is the momentum of the wave
𝑘
(2E)
h
2p
h
E
=p
mc2
(Since E = mc2)
= mv
=v
particle
c2
particle
vphase × vparticle = c2
But vg = vparticle
vphase × vg = c2
Expression for de-Broglie wavelength using group velocity:
We know, the group velocity vg =
dω
, where angular frequency ω = 2 and wave number k =
dk
1
2
λ
dω = 2d and dk = 2 d( λ )
Thus vg =
dω
=
dk
1
2d
1
2 d( )
λ
Or, d( λ ) =
d
vg
=
=
d
1
d( )
λ
d
vparticleg
-------- (1)
[since vg = vparticle]
Total energy of a particle moving under an applied potential V is given by,
1
E = 2 mv2 + V
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 9
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
de-Broglie related E = h to above expression
1
Then, h = mv2 + V
2
Assuming V as a constant potential and differentiating the above equation,
h d = m vparticle dv
mv
or, d = ( particle
) dv
h
Substituting this in equation (1),
1
m dv
d( λ) = h
Integrating,
1
mv
= h + constant
λ
i.e.,
1
λ
=
p
h
+ constant
Assuming constant of integration to be zero,
1
λ
=
or, λ =
p
h
𝐡
𝐩
+ constant
, the de-Broglie wavelength.
**************
VTU Model Question Paper
1 .a) 1) If the momentum of a particle is increased to four times, the de-Broglie wavelength is
i) become twice
ii) become for times
iii) become one-fourth
iv) become half
2) Blackbody radiation spectrum, maximum intensity is shifting towards
i) shorter wavelength ii) longer wavelength
iii) no change
iv) none of these
3) Group velocity of wave is equal to
i) V phase
ii) V particle
iii) Velocity of light
iv) none of these
4) de-Broglie wavelength of an electron accelerated by a potential of 60 V is
i) 1.85 Å
ii) 1.58 Å
iii) 1.589 Å
iv) 1.57 Å
(4 marks)
b) Describe Davisson-Germer experiment to prove the dual nature of matter waves. (8marks)
c) Explain phase velocity and Group velocity. Derive de-Broglie wavelength using Group velocity. (8 marks)
Dec 08/ Jan 09
1 a) 1) The de-Broglie wavelength associated with an electron of mass m and accelerated by a potential V is
h
√2mVe
h
h
i)
ii) ℎ
iii) Vem
iv) 2Vem
√2mVe
2) Davisson and Germer were the first to demonstrate:
i) The straight line propagation of light
ii) The diffraction of photons
iii) The effective mass of electron
iv) None of these
3) Electrons behaves as waves because they can be:
i) Deflected by an electric field
ii) Diffracted by a crystal
iii) Deflected by magnetic field
iv) They ionize a gas
4) In Davisson-Germer experiment, the hump is most prominent when the electron is accelerated by
i) 34 volts
ii) 54 volts
iii) 60 volts
iv) 80 volts
(04 Marks)
b) Define phase velocity and group velocity. Show that group velocity is same as particle velocity. (08 Marks)
c) Derive de-Broglie wavelength using Group velocity. (04 Marks)
d) Compare the energy of a photon with that of a neutron when both are associated with wavelength of 1 Å.
Given that mass of neutron is 1.678 × 10 -27 kg. (04 Marks)
June-July 2009
1) a) 1) An electron and a proton are accelerated through same potential. The ratio of de-Broglie wavelength
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 10
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
e/p is
i) 1
ii)
me
iii)
mp
mp
mp
iv) √
me
me
2) Wavefunction associated with a material particle is
i) Single valued
ii) Finite
iii) Continuous
iv) all the above
3) In a blackbody radiation spectrum, the maximum energy peaks shift towards the shorter wavelength side
with the increase in temperature. This confirms
i) Stefan’s law
ii) Wein’s law
iii) Rayleigh-Jean’s law
iv) Planck’s law
6
4) The group velocity of the particle is 3×10 m/s, whose phase velocity is
i) 6.06×106 m/s
ii) 3×1010 m/s
iii) 3 nm/s
iv) 1.5×1010 m/s (04 Marks)
b) Describe Davisson and Germer experiment for confirmation of deBroglie hypothesis. (08 Marks)
c) Explain phase and group velocity. Calculate the de-Broglie wavelength of a bullet of mass 5 gm moving
with a velocity 20 km/hr. (08 Marks)
Dec.09/Jan.10
1 a)1) Wien’s law is deduced from Planck’s radiation formula under the condition of
i) Very small wavelength and temperature
ii) Large wavelength and temperature
iii) Small wavelength and high temperature
iv) Large wavelength and small temperature
2) The Compton wavelength is given by
i) h/m0C2
ii) h2/m0C2
iii) h/m0C
iv) h2/2m0C
3) Which of the following relations can be used to determine de-Broglie wavelength associated with a
particle?
h
h
h
i)
ii) Vm
iii)
iv) All of these
√2mE
√2mVe
6
4) If the group velocity of a particle is 3×10 m/s, its phase velocity is
i) 100 m/s
ii) 3×106 m/s
iii) 3×108 m/s
iv) 3×1010 m/s (04 Marks)
b) What is Planck’s radiation law? Show how Wien’s law and Rayleigh-Jean’s law can be derived from it.
(06 Marks)
c) Define group velocity. Derive relation between group velocity and phase velocity. (06 Marks)
d) A fast moving neutron is found to be have a associated de-Broglie wavelength 2Å. Find its kinetic energy
and group velocity of the de-Broglie waves. (04 Marks)
May/June 2010
1 a) 1) In a blackbody radiation spectrum, the Wien’s distribution law is applicable only for
i) Longer wavelength
ii) Shorter wavelength
iii) Entire wavelength
iv) None of these
2) The de-Broglie wavelength associated with an electron of mass m and accelerated by a potential V is
h
√2mVe
h
h
i)
ii) ℎ
iii) Vem
iv) 2Vem
√2mVe
3) Electrons behaves as a wave because they can be
i) Diffracted by a crystal
ii) Deflected by magnetic field
iii) Deflected by electric field
iv) Ionise a gas
4) If the group velocity of de-Broglie wave is 4×108 m/sec, its phase velocity is
i) 12×108 m/sec
ii) 2.25×108 m/sec
iii) 5.33×108 m/sec iv) 1.33×108 m/sec (04 Marks)
b) Explain duality of matter waves.
(04 Marks)
c) Define phase velocity and group velocity. Show that group velocity is equal to particle velocity. (08 Marks)
d) Calculate the momentum of the particle and de-Broglie wavelength associated with an electron with a
kinetic energy of 1.5 keV. (04 Marks)
January 2011
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 11
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS
1 a) 1) Green light incident on a surface releases photoelectrons from the surface. If now blue light is incident
on the same surface, the velocity of electrons
i) increases
ii) decreases
iii) remains same
iv) becomes zero
2) Rayleigh-Jean’s theory of radiations agree with experimental results for
i) all wavelengths
ii) shorter wavelengths only
iii) longer wavelengths only
iv) middle order wavelengths only
3) The de-Broglie wavelength of an electron accelerated to a potential difference of 100 volts is
i) 1.2Å
ii) 10Å
iii) 100Å
iv) 12Å
4) The wave nature associated with electrons in motion was verified by
i) photoelectric effect
ii) Compton effect
iii) diffraction by crystals iv) Raman effect (04Marks)
b) State and explain de-Broglie’s hypothesis.
(04 Marks)
c) Define phase velocity and group velocity. Obtain the relation between group velocity and particle velocity.
Obtain the expression for de-Broglie wavelength using group velocity. (08 Marks)
d) Find the kinetic energy and group velocity of an electron with de-Broglie wavelength of 0.2 nm. (04 marks)
*************
Notes Compiled by: Dr. Santhosh D Shenoy, M.Sc., Ph.D.
www.bookspar.com | Website for Students | VTU NOTES | QUESTION PAPERS | NEWS | RESULTS Page 12