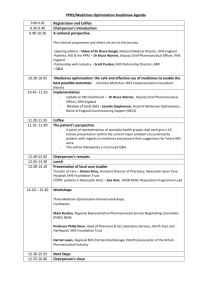
New proposal form February 2015
advertisement
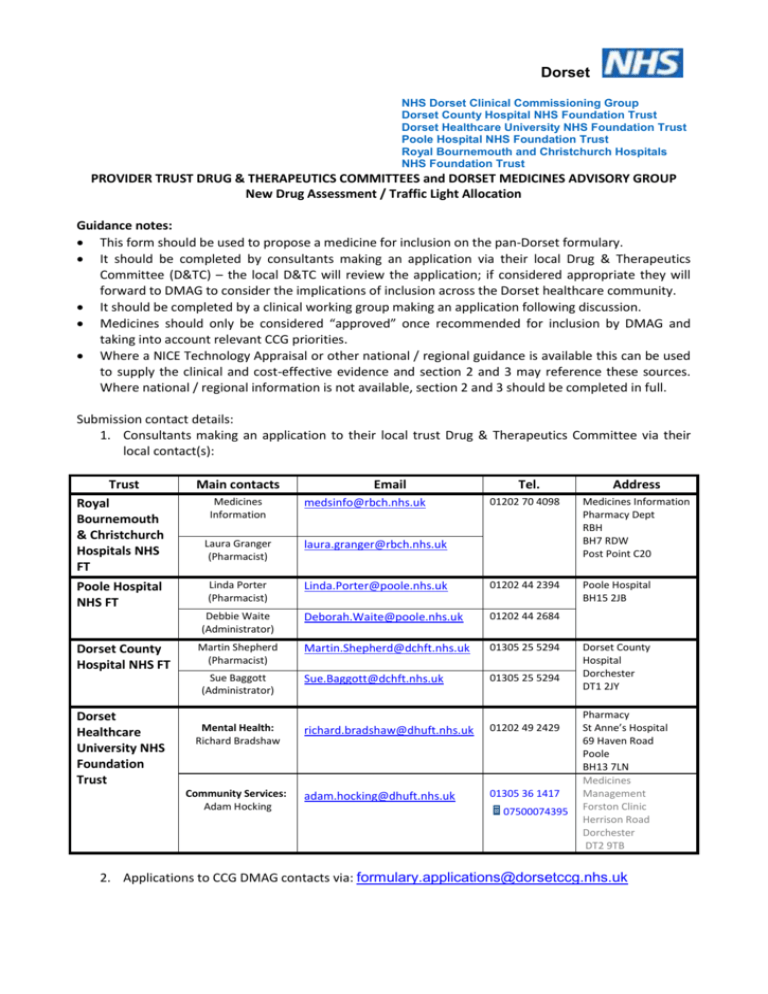
Dorset NHS Dorset Clinical Commissioning Group Dorset County Hospital NHS Foundation Trust Dorset Healthcare University NHS Foundation Trust Poole Hospital NHS Foundation Trust Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust PROVIDER TRUST DRUG & THERAPEUTICS COMMITTEES and DORSET MEDICINES ADVISORY GROUP New Drug Assessment / Traffic Light Allocation Guidance notes: This form should be used to propose a medicine for inclusion on the pan-Dorset formulary. It should be completed by consultants making an application via their local Drug & Therapeutics Committee (D&TC) – the local D&TC will review the application; if considered appropriate they will forward to DMAG to consider the implications of inclusion across the Dorset healthcare community. It should be completed by a clinical working group making an application following discussion. Medicines should only be considered “approved” once recommended for inclusion by DMAG and taking into account relevant CCG priorities. Where a NICE Technology Appraisal or other national / regional guidance is available this can be used to supply the clinical and cost-effective evidence and section 2 and 3 may reference these sources. Where national / regional information is not available, section 2 and 3 should be completed in full. Submission contact details: 1. Consultants making an application to their local trust Drug & Therapeutics Committee via their local contact(s): Trust Royal Bournemouth & Christchurch Hospitals NHS FT Main contacts Laura Granger (Pharmacist) laura.granger@rbch.nhs.uk Poole Hospital NHS FT Linda Porter (Pharmacist) Dorset County Hospital NHS FT Medicines Information Tel. Address 01202 70 4098 Medicines Information Pharmacy Dept RBH BH7 RDW Post Point C20 Linda.Porter@poole.nhs.uk 01202 44 2394 Poole Hospital BH15 2JB Debbie Waite (Administrator) Deborah.Waite@poole.nhs.uk 01202 44 2684 Martin Shepherd (Pharmacist) Martin.Shepherd@dchft.nhs.uk 01305 25 5294 Sue.Baggott@dchft.nhs.uk 01305 25 5294 richard.bradshaw@dhuft.nhs.uk 01202 49 2429 adam.hocking@dhuft.nhs.uk 01305 36 1417 Sue Baggott (Administrator) Dorset Healthcare University NHS Foundation Trust Email Mental Health: Richard Bradshaw Community Services: Adam Hocking medsinfo@rbch.nhs.uk 07500074395 Dorset County Hospital Dorchester DT1 2JY Pharmacy St Anne’s Hospital 69 Haven Road Poole BH13 7LN Medicines Management Forston Clinic Herrison Road Dorchester DT2 9TB 2. Applications to CCG DMAG contacts via: formulary.applications@dorsetccg.nhs.uk 1 GENERAL INFORMATION Generic (approved) name of medicine: Proprietary (brand) name of medicine: Therapeutic class : Manufacturer : Formulation(s) : Route(s) of administration : Proposed indication(s) National/regional guidance related to the medicine for this indication (see Appendix one): Has this drug or topic been discussed within a Clinical Commissioning Programme? What benefits does this drug offer over other therapy options in the same class? 2 EVIDENCE OF CLINICAL EFFICACY AND SAFETY Please list the pivotal studies that demonstrate the medicine’s safety and efficacy & what the principal outcome measures were, (copies of key papers should be provided – maximum of 3): Please state the number needed to treat (NNT) and number needed to harm (NNH) – if known : NNT= NNH= What are the known side effects? : Are there any safety issues regarding this medicine in comparison to existing medicines? : Are there any groups of patients in which this drug is contraindicated or where it should be used with caution? : Is there any long term safety data (if applicable)? : Are/should there be any restrictions on who should initiate treatment or administer the medicine? (if ‘yes’ please specify) e.g. No restrictions, Consultant only, Specialist Only, Use only according to guidelines : Has it been widely used elsewhere? (if ‘yes’ please specify) : Are there any clinically important drug interactions? (if ‘yes’ please specify): Are there any monitoring requirements? (if ‘yes’ please specify): Is this preparation part of a formal research project or clinical trial? (if ‘yes’ please give details) : 2 3. COST-EFFECTIVENESS Are there any published cost effectiveness analyses for the new medicine? Please provide information on the cost effectiveness of this medicine in terms of absolute risk reduction (ARR) and cost per QALY (if known/applicable)? : 4. FINANCIAL AND COMMISSIONING IMPLICATIONS OF ADDING TO FORMULARY Are there any other service implications? e.g. new equipment or processes (if ‘yes’ please specify which): What are the financial implications for the Dorset population? Are there off-set costs to be considered if this is added to formulary? What are the commissioning implications for provider Trusts and the CCG? Where does the balance of prescribing lie (see appendix 2) 5. BALANCE IN SECONDARY CARE PROPOSED TRAFFIC LIGHT CLASSIFICATION (see appendix 3) Green 6. BALANCE IN PRIMARY CARE Red Amber Not recommended DECLARATION OF INTEREST The lead clinician(s) responsible for completing this form, and/or providing information to the Medicines Advisory Group, are asked to declare and describe any involvement that they may have with the relevant pharmaceutical company, or with the manufacturers of any comparator products. Please declare any personal interests over the last 12 months. This involves payments (or other support) from any one company to an individual member or their spouse/partner/co-habittee or close relative. The main examples are consultancies, fee-paid work, travel grants or pharmaceutical company shares. (The amount of money involved does not have to be declared). If you have nothing to declare please type ‘None’: Please declare any non-personal interests over the last 12 months. This implies support from any one company for your unit or place of work. It may be financial or in kind, e.g. funding of a nurse, colleague, building or piece of equipment. (The amount of money involved does not have to be declared). If you have nothing to declare please type ‘None’: 3 7. DETAILS OF APPLICANT Name of requestor : Contact details (email address, telephone number, secretary etc.) : Speciality : Directorate : Trust: Date of submission : 8. DIRECTORATE SUPPORT – Supportive of application and aware of potential budgetary impact to directorate General Manager : Name (print) Signature Date Clinical Director : Name (print) 6. Signature Date APPROVAL Recommendation of local drug and therapeutics committee: Date of recommendation: Detail support of the D&TC: Are Provider Trusts or Primary Care supportive of this approach (where applicable)? Sponsor from local drug and therapeutics committee for this application: 9. OUTCOME OF MEDICINES ADVISORY GROUP DISCUSSIONS Notes: Date: Appendix 1 - Example of Organisations providing relevant resources on medicines All Wales Medicines Strategy Group Cochrane Library Drugs and therapeutics Bulletin Midlands Therapeutics Review and Advisory Committee National Institute for Health Research – Horizon Scanning Centre NICE NICE Medicines Information North East Treatment Advisory Group Regional Drug & Therapeutics Centre Scottish Intercollegiate Guidelines Network Scottish Medicines Consortium 4 Appendix 2 - Balance of responsibility – to assist with section 4 Criteria Primary Care Secondary Care (a) The disease/condition Familiar/common (b) Efficacy & effectiveness Initiation No special requirements Maintenance Benefit – measurable in primary care Dose titration Simple Withdrawal Lack of benefit – measurable in primary care Adverse events Predictable Chronic Knowledge base Monitoring-skills equipment Skill Equipment Training Large Specialist diagnosis required Intense initial monitoring Benefit measurable with specialist skills/equipment Complex depending on factors which can only be assessed in secondary care Lack of benefit – Requires specialist skills/equipment, close monitoring required Unpredictable adverse events Acute and life threatening Limited Available in primary care Specialised Available in primary care Available in primary care Specialist skills (c) (d) Safety Administration Unfamiliar/rare Appendix 3 - Definitions of traffic light categorisations in Dorset The “traffic light” system defines where responsibility for prescribing between primary and secondary care should lie through categorising individual drugs as red, amber or green. The “traffic light” system provides a framework for defining where clinical and therefore prescribing responsibility should lie through categorisation of individual drugs. The “traffic light” system is intended to provide a framework to consider the clinical responsibility and competency associated with the prescribing of a medicine and is not based on the cost of a medication. The list is advisory only (and can be changed by Dorset Medicines Advisory Group (DMAG) on request) but its existence should clarify expectations of prescribing responsibility. The DMAG recognises that there are some medicines with unresolved ‘traffic light status’ and others where prescribers may have concerns or disagreements arising from the reclassification of medicines. Please raise these issues with the formulary pharmacists of the employing Trust or the CCG senior pharmacists. The formulary pharmacists and DMAG will aim to resolve the issues in a timely manner. Following review of clinical data on efficacy, safety and cost-effectiveness by the DMAG and its sub-groups, drug treatments will either be recommended, following which they will receive a “traffic light” category as follows: Red - for secondary or tertiary care initiation and long-term maintenance of prescribing; 5 amber with shared care – drugs which are appropriate to be initiated and stabilised by a specialist in secondary or tertiary care, once stabilised the drug may be appropriate for responsibility to be transferred from secondary to primary care with the agreement of a GP and a formal ‘shared care’ agreement. o Initiation, prescribing, & monitoring until drug is stabilized is the responsibility of the specialist. o Specialist to request GP to take part in ongoing prescribing & associated clinical responsibility according to a ‘shared care guideline’, this may be prior to or following initiation of therapy o GP to respond to the specialist o Ongoing communication between primary and secondary care amber without shared care – drugs which may be initiated within a specialist service, which may be situated in primary, intermediate or secondary care, the drug may require stabilisation in the specialist service before transfer of prescribing to a primary care practitioner OR drugs which may be recommended by a specialist following a face-to face assessment or a telephone conversation with the patient’s GP and initiated by the GP o Initiation with 28 day supply (where possible) or recommendation is the responsibility of the specialist. Additional information regarding dosing and monitoring to be supplied by the specialist. o GP to respond to the specialist (where necessary). o Ongoing communication between primary and secondary care green - drugs which may be initiated, stabilised and maintained in a primary, secondary or tertiary care setting or, not recommended , that is where prescribing is not generally recommended in primary or secondary care. This category may be assigned to a drug where there is a lack of good clinical evidence for the drug, due to concerns over safety or due to the availability of more cost effective alternatives. This list will be reviewed regularly. Clinicians should not prescribe these drugs. This category includes all new black triangle ▼ drugs until they have been requested and formally assessed. For unlicensed medicines the prescriber, patient and GP should be aware of the unlicensed nature of the drug and reference to the protocol on the use of unlicensed drugs should be made. Commissioning issues may also need to be considered, for example, the prescribing of unlicensed medications should not generally be transferred to primary care, however off-label use may be suitable for transfer. Prescribers may wish to access the GMC guidance on prescribing off-label or unlicensed medications: http://www.gmc-uk.org/guidance/ethical_guidance/14327.asp Further detail of classifications Criteria for “Red” classification 1. Requiring specialist assessment to enable patient selection and initiation and continuation of treatment 2. Requiring long-term, on-going specialist monitoring of efficacy 3. Requiring long-term, on-going specialist monitoring of toxicity (because the side-effect profile necessitates rigorous supervision by the hospital consultant or, the full range of possible sideeffects, particularly long-term effects needs to be established (e.g cancer drugs) 6 4. Specifically designated as ‘hospital only’ by product licence (e.g. isotretinoin (Roaccutane®)) 5. That are new, or a new indication for an existing drug, that needs evaluation to be undertaken to establish place in therapy, with a recommendation that a formal review process be undertaken. 6. That are hospital indicated clinical trial materials 7. Use restricted by national guidance e.g NICE 8. Unlicensed / Off Label Drugs this includes unlicensed or named patient drugs, unlicensed doses or unlicensed indications for new▼drugs / drugs unfamiliar to primary care. Criteria for the “Amber with shared care” classification 1. Requiring specialist assessment to enable patient selection and initiation of treatment 2. Requiring specialist monitoring of efficacy, or that responsibility is clearly transferred to primary care 3. Requiring specialist monitoring of toxicity, or that responsibility is clearly transferred to primary care 4. That is rarely used, such that individual GPs are unlikely to see sufficient patients and acquire a working knowledge of the drug 5. NICE guidance 6. Products without a UK product licence would normally be classified as ‘red’ but may, in exceptional circumstances, be classified as ‘amber’. Shared Care Template A template for shared care agreements for drugs listed in the Shared care category of the panDorset formulary is available (link to be added). Shared care category drugs should be prescribed by a secondary care specialist or competent clinician to establish the patient on treatment, i.e. to monitor the patient’s response (efficacy, safety and appropriateness), adjust dosage and treat side effects. The patient needs to be stabilised and reviewed before asking the GP to take over clinical and prescribing responsibility. Each ‘shared care agreement’ will be individualised according to specific requirements of the drug. Shared care guidance should be sent and transfer agreed at least 2 weeks before the GP needs to prescribe the next period of treatment. The GP should consider the competencies required to manage and prescribe for the patient. Please note that primary care prescribers should not assume that drugs listed in the shared care group would attract “Near Patient Testing” payments under GMS Enhanced Services. This decision in relation to individual drugs is subject to local discussion with the CCG. Criteria for the “Amber without shared care” classification 1. Requiring an assessment by a specialist service, which may be situated in primary, intermediate or secondary care, to enable patient selection and initiation of treatment (this may be face to face or a telephone conversation with the patient’s GP) 2. Monitoring of efficacy can be undertaken in primary or secondary care 3. Monitoring of toxicity can be undertaken in primary or secondary care 4. Often used, such that individual GPs are likely to see sufficient patients and acquire a working knowledge of the drug. Criteria for the “Green” classification 1. Routinely used drugs with a high degree of universal familiarity 2. Initiation may be appropriate in primary, intermediate or secondary care 3. Offers significant benefit over existing treatment and that its use as a first, second or thirdline drug has been defined 7 Criteria for Not Recommended classification 1. Lack of data on effectiveness compared with standard therapy. 2. Lack of data on safety compared with standard therapy. 3. Known increase in risk of adverse events compared with standard therapy. 4. Lack of data on cost-effectiveness compared with standard therapy. 5. Less cost-effective than current standard therapy 6. NICE guidance which does not recommend the use of the drug 7. An interim measure pending review of the drug treatment It should be noted that there may be occasions where the use of a drug treatment that has been categorised as “not recommended” is considered appropriate. This should be managed by NHS Trusts and the Clinical Commissioning Group on an individual patient basis and with regard to appropriate commissioning arrangements. Second version: July 2014 8