UNIT 7 Metabolism and generation of ATP
advertisement

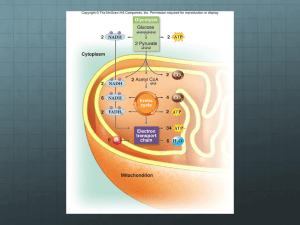
UNIT 7 Metabolism and generation of ATP Introduction to Unit Metabolism is the sum of biochemical processes that occur in living matter. Principally it involves the process of catabolism and anabolism. Simply stated, catabolism is the process of the breakdown or degradation of large molecules or polymer to smaller and simpler molecules. On the other hand, anabolism is the process of building up or synthesis of complex molecules. The various mechanisms of generating ATP starting with glucose which undergoes glycolysis will be explained. This is followed by citric acid cycle which produces intermediates and reductants which will then proceed to oxidative phosphorylation. Here, the role of mitochondria and electron transport system will be discussed as the ultimate generator of fuel molecules. Learning Outcomes At the end of this unit the students will be able to: 1. Explain the term metabolism and the main categories metabolism which is catabolism and anabolism. 2. Describe the process of glycolysis and pathways and determine the initial stages of ATP production and the enzymes involve in the conversion of glucose to pyruvate. 3. Describe and determine the pathways of citric acid cycle and oxidative phosphorylation 4. Relate the role of mitochondria as the factory for the production of ATP 101 TOPIC 1 : Metabolism and their regulation Main Points 1.1 Metabolism is the process involving catabolism and anabolism. Catabolism is defined as the process of degradation or breakdown of complex substances to simpler molecules whereas anabolism is the process of synthesizing of complex organic molecules from simple and small molecules. The function of catabolism is to generate energy from fuel molecules. 1.2 Both catabolism and anabolism occur in three stages of complexity – stage 1, the interconversion of polymers and complex lipids with monomeric intermediates; stage 2, the interconversion of monomeric sugars, amino acids and lipids with still simpler organic compounds and stage 3, the ultimate degradation to, or synthesis from inorganic compounds such as CO2, H2O and NH3. These stages of complexity are shown below. 102 1.3 Metabolic pathways for biosynthesis and degradation (anabolism and catabolism) are not simple reversals of each other. Though there may be individual reactions that are reversals of each other, there are at least some enzymes that are different, some regulation systems that are opposed to each other, and some metabolites that vary between the two. Pathways may even occur in separate cellular compartments. For example, fatty acid synthesis takes place in cytosol, whereas fatty acid oxidation takes place in mitochondria. 1.4 The existence of separate pathways is important for two reasons. First, to proceed in a particular direction, a pathway must be exergonic (energy is released so G is negative)) in that direction. 103 If a pathway is strongly exergonic overall, then simple reversal of that pathway is just as strongly endergonic (require energy so G is positive) under the same conditions. 1.5 Second, and equally important, is the need to control the flow of metabolites in relation to the bioenergetic status of a cell. When ATP levels are high, there is less need for carbon to be oxidized in the citric acid cycle. At such times the cell can store carbon as fats and carbohydrates, so fatty acid synthesis, gluconeogenesis, and related pathways come into play. When ATP levels are low, the cell must mobilize stored carbon to generate substrates for the citric acid cycle, so carbohydrate and fat breakdown must occur. Using separate pathways for the biosynthetic and degradative processes is crucial for control, so conditions that activate one pathway tend to inhibit the opposite pathway and vice versa. 1.6 Biosynthetic pathways require energy to operate and degradative pathways yield less energy than biosynthetic pathways require. Thus, if both pathways are running at the same time and place, they will go in circles, producing no useful metabolites while consuming energy. Cells have control systems to activate one member of a catabolic/anabolic pair, while inhibiting the other. 104 1.7 An overview of metabolism is shown above which also include the role of photosynthesis which will be discussed in a separate unit (See Unit 8 of this module). 1.8 In biological oxidations, reactions occur without a large increase in temperature and with capture of some of the free energy as chemical 105 energy. Metabolic energy capture occurs largely through the synthesis of ATP, a molecule designed to provide energy for biological work. The capture of energy is quite efficient. In the catabolism of glucose, for example, about 40% of the 2870 kJ/mol of energy released is used to drive the synthesis of ATP from ADP and Pi. 1.9 Most biological oxidations do not involve direct transfer of electrons from a substrate directly to oxygen. Instead, a series of coupled oxidation-reduction reactions occurs, with the electrons passed to intermediate electron carriers such as NAD+ before they are finally transferred to oxygen. Because metabolic energy comes primarily from oxidative reactions, the more highly reduced a substrate, the higher its potential for generating biological energy. Thus, combustion of fat provides more heat energy than combustion of an equivalent mass of carbohydrate. 1.10 Reducing equivalents can be defined as 1 mole of hydrogen atoms (one proton and one electron per H atom). For example, two reducing equivalents are used in the reduction of one half mole of oxygen to water: 1/2 O2 + 2e- + 2H+ -> H2O Remember that the breakdown of complex organic compounds yields both energy and reducing equivalents, and the biosynthesis of such compounds utilizes both. 1.11 ATP is used to provide energy in a wide variety of metabolic reactions and is universal among cells. An array of enzymes preferentially binds ATP and uses its free energy of hydrolysis to drive endergonic reactions. Hydrolysis of either phosphoanhydride bond in ATP has a G of about -31 kJ/mol. Be aware, however, that utilization of that energy to drive endergonic reactions usually does NOT involve hydrolysis of ATP. 1.12 Instead, ATP breakdown is usually coupled with a thermodynamically unfavorable reaction. In glycolysis, for example, ATP energy is used to synthesize glucose-6-phosphate from glucose. In this case, the phosphate is transferred directly from ATP to glucose to form glucose-6-phosphate. Because ATP can transfer a phosphate group, we say that ATP has a high "phosphoryl group transfer potential" rather than calling it a high energy 106 compound. The phosphate anhydride bonds of ATP, ADP, or pyrophosphate have relatively high values. 1.13 Mechanisms for controlling metabolic pathways are varied in cells. Some include the following: Control of enzyme levels The concentrations of different enzymes vary widely in cellular extracts. Enzyme levels are controlled in large part by controlling the enzyme's rate of synthesis. Enzyme synthesis can often be induced or repressed by the presence or absence of certain metabolites. The rate of enzyme degradation can also be a factor in controlling enzyme levels. 1.14 Compartmentation Eukaryotic cells contain many different organelles and enzymes are distributed unevenly throughout them. For example, RNA polymerases are found in the nucleus and the nucleolus, where DNA transcription occurs. Enzymes of the citric acid cycle, on the other hand, are found in the mitochondria. Enzymes of fatty acid synthesis are found in the cytoplasm, but enzymes for fatty acid degradation are found in mitochondria. 1.15 Hormonal regulation Cells must respond to changes in the environment and/or to signals from other cells. The process of transmitting this information from outside of the cell to inside the cell is called signal transduction. One of the extracellular messengers that carry the information include hormones. Hormones are substances synthesized in specialized cells and carried via the blood to remote target cells. There the hormones interact with specific receptors, resulting in specific metabolic changes in the target cell. (See Unit 9 for further information). 107 Main Points TOPIC 2: Glycolysis and their regulation 2.1 One of the central metabolic pathways is glycolysis. It involves degradation of carbohydrates, in either aerobic or anaerobic cells. Pyruvate, the product of glycolysis, is handled differently by anaerobic (fermentation) and aerobic pathways. The first stage of glucose metabolism which produces pyruvate is called glycolisis and yields two ATP. 2.2 The complete aerobic oxidation of glucose to carbon dioxide and water involves glycolisis, the citric acid cycle and oxidative phosphorylation yielding the energy equivalent of 32 ATP. Under aerobic condition the main purpose of glycolisis is to feed pyruvate into citric acid cycle, where further metabolic step will yield considerably more ATP. 2.3 During intense physical activity, the body metabolizes carbohydrates faster than the aerobic process. Under anaerobic condition, the glucose will first be metabolised to pyruvate which is subsequently converted to lactic acid in the contracting muscle. The two ATPs generated by glycolisis serve as an additional energy source under this condition. 2.4 Anaerobic pathways lead to a variety of products, including lactate and ethanol while aerobic pathways lead to acetyl-CoA and ultimately to carbon dioxide in the citric acid cycle. 108 2.5 There are ten steps in glycolysis, the first five reactions is considered as the energy investment phase in which sugar phosphates are synthesized at the expense of 2 moles of ATP, converted to ADP. The 6-carbon glucose is split into two 3-C sugar phosphates. The last five reactions represent the energy generation phase in which triose phosphates are converted to energy-rich compounds. These transfer of 4 moles of phosphate to ADP leading to 4 ATPs. The net yield is 2 moles of ATP and 2 moles of pyruvate. Two moles of reducing equivalents are also produced in the form of NADH. 109 2.6 Glycolysis is the first step in the complete oxidation of glucose to CO2 and water. The second step is the oxidation of pyruvate to acetyl CoA and the final process is the oxidation of acetyl group carbons in the citric acid cycle (Kreb’s cycle or tricarboxylic acid cycle). Glycolysis is seen as both and anabolic and catabolic pathway that not only provides energy fuels but also intermediates for biosynthesis. 110 Main Points TOPIC 3: CITRIC ACID CYCLE AND AND ITS REGULATION 3.1 The citric acid cycle is a central metabolic pathway which generates NADH and FADH2 for use in electron transport It also produces GTP via substrate-level phosphorylation. Many metabolic processes use intermediates of the citric acid cycle in their pathways. The cyclic process is generally considered to "begin" with addition of acetyl-CoA to oxaloacetate to form citrate. Remember, however, that the pathway is cyclic. 3.2 The fate of carbon from acetyl CoA is shown below. 111 3.3 In phase 1 of the citric acid cycle or tricarboxylic acid (TCA) cycle, it involves the introduction and loss of two carbon atoms. The reactions involve in this phase are: • 1. Acetyl-CoA + Oxaloacetate + H2O <=> Citrate + CoASH + H+ Enzyme: Citrate Synthase Go’ = -32.2 kJ/mol • 2. Citrate <=> cis-Aconitate + H2O <=> Isocitrate Enzyme: Aconitase Go’ = +6.3 kJ/mol • 3. Isocitrate + NAD+ <=> -Ketoglutarate + CO2 + NADH Enzyme: Isocitrate Dehydrogenase Go’ = -20.9 kJ/mol • 4. -Ketoglutarate + NAD+ + CoASH <=> Succinyl-CoA + CO2 + NADH Enzyme: Ketoglutarate Dehydrogenase Complex Go’ = -33.5 kJ/mol The second phase of the cycle is regeneration of oxaloacetate and the reactions involved are: • 5. Succinyl-CoA + Pi + GDP <=> Succinate + GTP + CoASH Enzyme: Succinyl-CoA Synthetase Go’ = -2.9 kJ/mol • 6. Succinate + FAD (enzyme bound) <=> Fumarate + FADH2 Enzyme: Succinate Dehydrogenase Go’ = 0 kJ/mol • 7. Fumarate + H2O <=> L-Malate Enzyme: Fumarate Hydratase Go’ = -3.8 kJ/mol • 8. L-Malate + NAD+ <=> Oxaloacetate + NADH + H+ Enzyme: Malate Dehydrogenase Go’ = +29.7 kJ/mol This completes the cycle whereby oxaloacetate is re-generated ready to react with in-coming acetyl CoA generated by glycolysis. The generated during the TCA cycle are: Acetyl-CoA, intermediates Oxaloacetate, that Citrate, were cis- Aconitate, D-Isocitrate, α-ketoglutarate, Succinyl-CoA, Succinate, Fumarate and LMalate. 3.3 Citric acid cycle is a source of biosynthetic intermediates, as well as a route for generating metabolic energy. Regulation of the cycle is somewhat more complex than if it were solely an energy-generating pathway. The citric acid cycle is regulated in two primary ways, by controlling the entry of fuel into the cycle and by controlling key reactions within the cycle. 3.4 The entry of fuel into the cycle is controlled by substrate level regulation. Substrate level regulation occurs by limiting amounts of acetyl-CoA, oxaloacetate, 112 citrate, and NAD+/NADH. The latter is the most important and common substratelevel regulation. NAD+ is an essential substrate for three enzymes in the cycle as well as the pyruvate dehydrogenase complex. Conditions which reduce NAD+ concentration include lack of oxygen, because the electron transport system which converts NADH back to NAD+ usually depends upon oxygen. 3.5 Key reactions within the cycle: Movement of materials through the citric acid cycle is also regulated allosterically. Enzymes suspceptible to allosteric regulation include isocitrate dehydrogenase and -ketoglutarate dehydrogenase. Isocitrate dehydrogenase: Activated by ADP, inhibited by NADH (apart from substrate level regulation of NAD+) . Phosphorylation of one serine residue in the enzyme prevents binding of isocitrate. -ketoglutarate dehydrogenase: Inhibited by succinyl-CoA and NADH. 3.6 To summarize, the citric acid cycle is responsive to the energy state of the cell, through allosteric activation of isocitrate dehydrogenase by ADP; to the redox state of the cell, through flux rate limitation caused when intramitochondrial [NAD +] decreases; and to the availability of energy-rich compounds, through inhibition of relevant enzymes by acetyl-CoA or succinyl-CoA. 113 Main Points TOPIC 4 MITOCHONDRIA STRUCTURE AND OXIDATIVE PHOSPHORYLATION 4.1 Metabolic oxidations generate reduced electron carriers, such as NADH and FADH2. Oxidation of these electron carriers in the mitochondrion generates most of the energy needed for ATP synthesis. Most vertebrate cells contain several hundred mitochondria, but the number can be as low as 1 and as high as 100,000. Mitochondria has an outer membrane, an inner membrane, an intermembrane space, and a matrix, located within the inner membrane. The outer membrane is porous, whereas the inner membrane is much tighter, serving as a barrier to many biological metabolites. 4.2 The inner membrane is highly folded into cristae, which project into, and often nearly through the interior of the mitochondrion. Understanding the function of the inner mitochondrial membrane is a key to understanding how the mitochondrion works. Processes occurring inside the mitochondrial matrix include pyruvate oxidation, fatty acid oxidation, amino acid metabolism, and the citric acid cycle. Furthermore, respiratory proteins are bound to the inner membrane, so the density of cristae corresponds to the respiratory activity of a cell. For example, mitochondria in heart muscle cells (high rates of respiration) are densely packed with cristae. 4.3 Embedded within the inner membrane are the protein electron carriers (primarily) cytochromes, which constitute the respiratory chain. They are assembled in the form of five multiprotein complexes, named I, II, III, IV, and V. Smaller carriers, such as coenzyme Q and cytochrome c, also participate in carrying electrons. The diagram below shows metabolic reactions occurring inside the mitochondrial matrix and movement of electrons through the complexes in the inner mitochondrial membrane. Note that electrons are ultimately donated with protons to oxygen to form water. 114 4.4 When a compound is oxidized, it loses electrons. In biological systems, electrons from oxidation are generally transferred to electron-carrying molecules, such as NAD+ or FAD to form NADH and FADH2, respectively. Note that NAD+ and FAD are the oxidized forms of the molecules and NADH and FADH 2 are the reduced forms. Thus, biological oxidations generate reduced electron carriers. 4.5 Reduced electron carriers donate their electrons to acceptor molecules and become reoxidized in the process. The acceptor molecules are reduced because the oxidation of one species (e.g., the reduced electron carrier) cannot occur without the simultaneous reduction of another species (e.g., the acceptor molecule). 4.6 The inner mitochondrial membrane contains a complex called Complex I that accepts electrons from NADH. In the process, the complex is reduced and NAD+ is re-formed. Electrons from FADH2 are transferred to a different mitochondrial complex, called Complex II. The previous diagram depicts the movement of electrons from Complex I and Complex II through the other electron carriers in the inner mitochondrial membrane. The complexes of the inner mitochondrial membrane that shuttle electrons are called the electron transport system (ETS). 115 4.7 After electrons pass through Complex IV, they are donated to oxygen along with protons to form water (see diagram below). As electrons move through complexes I, III, and IV of the ETS, protons are "pumped" from the mitochondrial matrix to the intermembrane space. This creates a potential energy source, with a high concentration of protons in the intermembrane space and a relatively low concentration of protons in the mitochondrial matrix. 4.8 Pumps require energy to function. The "pumps" of the ETS chain derive their energy to transport protons from oxidation/reduction (called redox) reactions that occur as electrons move from one complex to another. To quantitate the amount of energy in these transfers, the standard reduction potential can be used. 4.9 Oxidative phosphorylation is a process where the energy of biological oxidation is ultimately converted to the chemical energy of ATP. Movement of electrons through the electron transport system (ETS) causes protons to be pumped from the mitochondrial matrix of a eukaryotic cell to the intermembrane space. The difference in potential created by movement of the charged protons as well as the concentration gradient created by the pumping provides the energy source for making ATP in the mitochondrion. This process is called oxidative phosphorylation. 116 4.10 It occurs as a result of protons moving through Complex V. The ETS pumps protons out of the mitochondrial matrix to the intermembrane space. When needed, protons flow back through Complex V and turn molecular turbines to make ATP. The movement of protons and NAD+ through the ETS is shown below. 4.11 The energy yields, starting with glucose and its complete oxidation will generate the following amount of NADH, FADH and ATP. 117