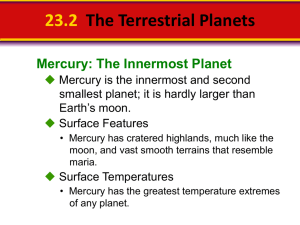
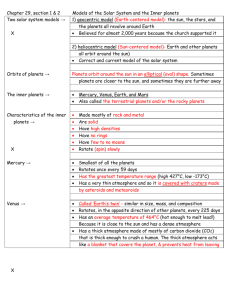
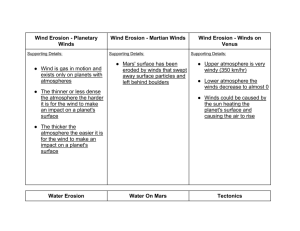
Data Analysis: Teacher Directed Part 1 - Science - Miami
advertisement

Miami-Dade County Public Schools Division of Academics Required ESSENTIAL Laboratory Activities For the Middle School M/J Comprehensive Science 3 REVISED May 2014 EL8_2014 1 THE SCHOOL BOARD OF MIAMI-DADE COUNTY, FLORIDA Ms. Perla Tabares Hantman, Chair Dr. Lawrence S. Feldman, Vice-Chair Dr. Dorothy Bendross-Mindingall Ms. Susie V. Castillo Mr. Carlos L. Curbelo Dr. Wilbert “Tee” Holloway Dr. Martin Karp Dr. Marta Pérez Ms. Raquel A. Regalado Ms. Krisna Maddy Student Advisor Mr. Alberto M. Carvalho Superintendent of Schools Ms. Marie Izquierdo Chief Academic Officer Office of Academics and Transformation Dr. Maria P. de Armas Assistant Superintendent Division of Academics, Accountability, and School Improvement Mr. Cristian Carranza Administrative Director Division of Academics, Accountability & School Improvement Dr. Ava D. Rosales Executive Director Department of Mathematics and Science EL8_2014 2 Table of Contents Introduction ................................................................................................................................... 4 Annually Assessed Benchmarks .................................................................................................. 5 Materials List ................................................................................................................................. 8 Lab Roles ..................................................................................................................................... 10 Safety Information and Contract .............................................................................................. 11 Pre-Lab Safety Worksheet and Approval Form ...................................................................... 12 Parts of a Lab Report ................................................................................................................. 13 Experimental Design Diagram ................................................................................................... 17 Claim Evidence Reasoning ......................................................................................................... 18 Engineering Design Process ....................................................................................................... 19 Essential Labs Experimental Design: Pasta Strength (Topic 1)……………………………………………….20 What’s the Matter? Inquiry Lab(Topic 2).………………………………………….…………24 Physical Changes and Chemical Changes Inquiry Lab(Topic 3)………….…………………32 Atomic Modeling (Topic 4)……………………………………………………………….……..39 Periodic Table of Elements (Topic 5)……………………………………………………….….44 Clay Elements, Compounds/Molecules (Topic 6)………………………………………….…..49 Investigating the Effect of Light Intensity on Photosynthesis (Topic 7)………………….…..55 Conservation of Mass (Topic 7) ………………………………………………………….……..61 Carbon Cycle Game (Topic 8)…………………………………………………………………..66 Scale of the Universe Modeling Activity (Topic 9)……………………………………….……79 Star Bright Apparent Magnitude Lab (Topic 10)……………………………………………..82 The Martian Sun-Times (Topic 11)…………………………………………………………….86 What Causes the Seasons? (Topic 12)........................................................................................96 Additional Resources Density of Rocks with Differentiated Lab………………………………………………………...103 Density of Rocks (Revised by University of Miami Science Made Sensible Fellows)………….109 Precipitating Bubbles with Differentiated Lab ........................................................................ .122 Greenhouse Gases in a Bottle with Differentiated Lab ............................................................ 134 Imaginary Alien Life-forms with Differentiated Lab (Adaptations and Punnett Square)........ 138 Planetary Exploration and Extreme Life Forms with Differentiated Lab ............................. 155 Revised by University of Miami Science Made Sensible Fellows EL8_2014 3 Introduction The purpose of this packet is to provide the M/J Comprehensive Science 3 and Grade 8 teachers with a list of minimum basic laboratories and hands-on activities that students should experience in class. Each activity is aligned with the Next Generation Sunshine State Standards (NGSSS). Emphasis has been placed on those hands-on activities that are aligned to the Annually Assessed Benchmarks, which are assessed in the Florida Comprehensive Assessment Test 2.0 (FCAT 2.0), administered in grade eight (8). In most cases, the activities were designed as simple as possible without the use of advanced technological equipment to make it possible for all teachers to use these activities. All activities and supplements (i.e., Parts of a Lab Report) can be modified, if necessary, to fit the needs of an individual class and/or student ability. This document is intended to be used by science departments in M-DCPS so that all science teachers can work together, plan together, and rotate lab materials among classrooms. Through this practice, all students and teachers will have the same opportunities to participate in these experiences and promote discourse among learners which are the building blocks of authentic learning communities. Acknowledgement: M-DCPS Department of Mathematics and Science would like to acknowledge the efforts of the teachers who worked arduously and diligently on the preparation of this document. EL8_2014 4 Annually Assessed Benchmarks Next Generation Sunshine State Standard (NGSSS) SC.8.N.1.1 Define a problem from the eighth grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of various types, such as systematic observations or experiments, identify variables, collect and organize data, interpret data in charts, tables, and graphics, analyze information, make predictions, and defend conclusions. (Also assesses SC.6.N.1.1, SC.6.N.1.3, SC.7.N.1.1, SC.7.N.1.3, SC.7.N.1.4, SC.8.N.1.3, and SC.8.N.1.4.) (Cognitive Complexity Level 3: Strategic Thinking and Complex Reasoning) SC.7.N.1.2 Differentiate replication (by others) from repetition (multiple trials). (Also assesses SC.6.N.1.2, SC.6.N.1.4, and SC.8.N.1.2.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.7.N.1.5 Describe the methods used in the pursuit of a scientific explanation as seen in different fields of science such as biology, geology, and physics. (Also assesses SC.7.N.3.2, SC.8.N.1.5, and SC.8.E.5.10.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.6.N.2.2 Explain that scientific knowledge is durable because it is open to change as new evidence or interpretations are encountered. (Also assesses SC.7.N.1.6, SC.7.N.1.7, SC.7.N.2.1, and SC.8.N.1.6.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.7.N.3.1 Recognize and explain the difference between theories and laws and give several examples of scientific theories and the evidence that supports them. (Also assesses SC.6.N.3.1 and SC.8.N.3.2.) (Cognitive Complexity Level 3: Strategic Thinking and Complex Reasoning) SC.8.E.5.3 Distinguish the hierarchical relationships between planets and other astronomical bodies relative to solar system, galaxy, and universe, including distance, size, and composition. (Also assesses SC.8.E.5.1 and SC.8.E.5.2.) (Cognitive Complexity Level 3: Strategic Thinking and Complex Reasoning) SC.8.E.5.5 Describe and classify specific physical properties of stars: apparent magnitude (brightness), temperature (color), size, and luminosity (absolute brightness). (Also assesses SC.8.E.5.6.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.8.E.5.7 Compare and contrast the properties of objects in the Solar System including the Sun, planets, and moons to those of Earth, such as gravitational force, distance from the Sun, speed, movement, temperature, and atmospheric conditions. (Also assesses SC.8.E.5.4 and SC.8.E.5.8.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.8.E.5.9 Explain the impact of objects in space on each other including: 1. the Sun on the Earth including seasons and gravitational attraction 2. the Moon on the Earth, including phases, tides, and eclipses, and the relative position of each body. (Cognitive Complexity Level 3: Strategic Thinking and Complex Reasoning) SC.7.E.6.2 Identify the patterns within the rock cycle and events (plate tectonics and mountain building). (Also assesses SC.6.E.6.1, SC.6.E.6.2, and SC.7.E.6.6.) relate them to surface events (weathering and erosion) and subsurface events (plate tectonics and mountain building). (Also assesses SC.6.E.6.1, SC.6.E.6.2, and SC.7.E.6.6.) (Cognitive Complexity Level 3: Strategic Thinking and Complex Reasoning) SC.7.E.6.4 Explain and give examples of how physical evidence supports scientific theories that Earth has evolved over geologic time due to natural processes. (Also assesses SC.7.E.6.3.) (Cognitive Complexity Level 3: Strategic Thinking and Complex Reasoning) EL8_2014 5 SC.7.E.6.5 Explore the scientific theory of plate tectonics by describing how the movement of Earth’s crustal plates causes both slow and rapid changes in Earth’s surface, including volcanic eruptions, Earthquakes, and mountain building. (Also assesses SC.7.E.6.1 and SC.7.E.6.7.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.6.E.7.4 Differentiate and show interactions among the geosphere, hydrosphere, cryosphere, atmosphere, and biosphere. (Also assesses SC.6.E.7.2, SC.6.E.7.3, SC.6.E.7.6, and SC.6.E.7.9.) (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) SC.6.E.7.5 Explain how energy provided by the Sun influences global patterns of atmospheric movement and the temperature differences between air, water, and land. (Also assesses SC.6.E.7.1.) (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. (Also assesses SC.8.P.8.3.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.8.P.8.5 Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter. (Also assesses SC.8.P.8.1, SC.8.P.8.6, SC.8.P.8.7, SC.8.P.8.8, and SC.8.P.8.9.) (Cognitive Complexity Level 1: Recall) SC.8.P.9.2 Differentiate between physical changes and chemical changes. (Also assesses SC.8.P.9.1 and SC.8.P.9.3.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.7.P.10.1 Illustrate that the Sun’s energy arrives as radiation with a wide range of wavelengths, including infrared, visible, and ultraviolet, and that white light is made up of a spectrum of many different colors. (Also assesses SC.8.E.5.11.) (Cognitive Complexity Level 1: Recall) SC.7.P.10.3 Recognize that light waves, sound waves, and other waves move at different speeds in different materials. (Also assesses SC.7.P.10.2.) (Cognitive Complexity Level 1: Recall) SC.7.P.11.2 Investigate and describe the transformation of energy from one form to another. (Also assesses SC.6.P.11.1 and SC.7.P.11.3.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.7.P.11.4 Observe and describe that heat flows in predictable ways, moving from warmer objects to cooler ones until they reach the same temperature. (Also assesses SC.7.P.11.1.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.6.P.13.1 Investigate and describe types of forces including contact forces and forces acting at a distance, such as electrical, magnetic, and gravitational. (Also assesses SC.6.P.13.2 and SC.8.P.8.2.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.6.P.13.3 Investigate and describe that an unbalanced force acting on an object changes its speed, or direction of motion, or both. (Also assesses SC.6.P.12.1.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.6.L.14.1 Describe and identify patterns in the hierarchical organization of organisms from atoms to molecules and cells to tissues to organs to organ systems to organisms. (Cognitive Complexity Level 1: Recall) EL8_2014 6 SC.6.L.14.2 Investigate and explain the components of the scientific theory of cells (cell theory): all organisms are composed of cells (single-celled or multi-cellular), all cells come from preexisting cells, and cells are the basic unit of life. (Also assesses SC.6.L.14.3.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.6.L.14.4 Compare and contrast the structure and function of major organelles of plant and animal cells, including cell wall, cell membrane, nucleus, cytoplasm, chloroplasts, mitochondria, and vacuoles. (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.6.L.14.5 Identify and investigate the general functions of the major systems of the human body (digestive, respiratory, circulatory, reproductive, excretory, immune, nervous, and musculoskeletal) and describe ways these systems interact with each other to maintain homeostasis. (Also assesses SC.6.14.6.) (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) SC.6.L.15.1 Analyze and describe how and why organisms are classified according to shared characteristics with emphasis on the Linnaean system combined with the concept of Domains. (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) SC.7.L.15.2 Explore the scientific theory of evolution by recognizing and explaining ways in which genetic variation and environmental factors contribute to evolution by natural selection and diversity of organisms. (Also assesses SC.7.L.15.1 and SC.7.L.15.3.) (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) SC.7.L.16.1 Understand and explain that every organism requires a set of instructions that specifies its traits, that this hereditary information (DNA) contains genes located in the chromosomes of each cell, and that heredity is the passage of these instructions from one generation to another. (Also assesses SC.7.L.16.2 and SC.7.L.16.3.) (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) SC.7.L.17.2 Compare and contrast the relationships among organisms such as mutualism, predation, parasitism, competition, and commensalism. (Also assesses SC.7.L.17.1 and SC.7.L.17.3.) (Cognitive Complexity Level 2: Basic Application of Skills and Concepts) SC.8.L.18.4 Cite evidence that living systems follow the Laws of Conservation of Mass and Energy. (Also assesses SC.8.L.18.1, SC.8.L.18.2, and SC.8.L.18.3.) (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) EL8_2014 7 MATERIALS LIST Each list corresponds to the amount of materials needed per station (whether one student or a group of students uses the station). Lab Aprons and goggles should be assigned to each student on all labs requiring mixtures of chemicals. Pasta Stength Ruler 50-100 Pennies “What’s the Matter?” Inquiry Lab Part 1 – Separating Mixture Mystery Mixture (sugar, sand, water, wood chips, and iron fillings or staples) Hot Plate Triple Beam Balance Styrofoam/Plastic Cup and string to make a handle (see picture) 5 Strands of uncooked pasta (provide variety) Tape Coffee Filter Magnet Beaker Thermometer Graduated Cylinder Part 2 – Physical & Chemical Changes Test tubes Magnet Wooden Splints Thermometer Water Baking Soda Effervescent tablet Salt Magnifying glass Hot Plate Iron Fillings Physical Change and Chemical Changes in Matter Materials (per group) Beakers (2) Test tubes (6) Test tube rack Stirrers Water Milk Cabbage Juice Baking Soda Calcium Chloride Atomic Models Materials Handout & Periodic Table of Elements Clay Elements, Molecules and Compounds Materials: Paper Towel Toothpicks EL8_2014 Graduated Cylinder Small beaker Vinegar Thermometer Vinegar Modeling Clay Colored pencils 8 Investigating the Effect of Light intensity on Photosynthesis Materials: Test tube Source of bright light Sodium bicarbonate solution Watch or clock with second indicator 400-mL beaker Plastic gloves Freshly cut sprig of an evergreen (such as Hand lens yew) or elodea Forceps Conservation of Mass Materials: Graduated Cylinder Erlenmeyer Flask Balloon Baking Soda Triple Beam Balance Spoon Carbon Cycle Game 7 Dice 7 Station Signs 7 Station Movement Directions Carbon Cycle Passport for Each Student Carbon Atom Model for Each Student Blank Bar Graph for Each Student Scale of the Universe Modeling Activity Materials (Suggested, but not limited to) Modeling clay String Different sized balls Markers Star Bight Apparent Magnitude Lab Materials (per group): 3 pencils 1 meter stick The Martian Sun-Times Worksheets Computer with Internet access meter stick markers or colored pencils metric ruler scissors What Causes the Seasons? Globe of the Earth Tape Metric ruler Thermometer EL8_2014 Paper Scissors Tape Balloons Straws 2 flashlights receipt paper rolls (adding machine tape) or old VHS tape Various spherical objects of different sizes (basketball, marbles, softball, tiny beads, soccer ball) Lamp with 100-watt bulb Ring stand and utility clamp 20-cm Length of string 9 Lab Roles and Their Descriptions Cooperative learning activities are made up of four parts: group accountability, positive interdependence, individual responsibility, and face-to-face interaction. The key to making cooperative learning activities work successfully in the classroom is to have clearly defined tasks for all members of the group. An individual science experiment can be transformed into a cooperative learning activity by using these lab roles. Project Director (PD) The project director is responsible for the group. Roles and responsibilities: Reads directions to the group Keeps group on task Is the only group member allowed to talk to the teacher Shares summary of group work and results with the class Materials Manager (MM) The materials manager is responsible for obtaining all necessary materials and/or equipment for the lab. Roles and responsibilities: The only person allowed to be out of his/her seat to pick up needed materials Organizes materials and/or equipment in the work space Facilitates the use of materials during the investigation Assists with conducting lab procedures Returns all materials at the end of the lab to the designated area Technical Manager (TM) The technical manager is in charge of recording all data. Roles and responsibilities: Records data in tables and/or graphs Completes conclusions and final summaries Assists with conducting the lab procedures Assists with the cleanup Safety Director (SD) The safety director is responsible for enforcing all safety rules and conducting the lab. Roles and responsibilities: Assists the PD with keeping the group on-task Conducts lab procedures Reports any accident to the teacher Keeps track of time Assists the MM as needed. When assigning lab groups, various factors need to be taken in consideration; Always assign the group members preferably trying to combine in each group a variety of skills. For example, you can place an “A” student with a “B”, a “C” and a “D” or an “F” student. Evaluate the groups constantly and observe if they are on task and if the members of the group support each other in a positive way. Rotation of lab groups and members throughout the year is encouraged. EL8_2014 10 Laboratory Safety Rules: Know the primary and secondary exit routes from the classroom. Know the location of and how to use the safety equipment in the classroom. Work at your assigned seat unless obtaining equipment and chemicals. Do not handle equipment or chemicals without the teacher’s permission. Follow laboratory procedures as explained and do not perform unauthorized experiments. Work as quietly as possible and cooperate with your lab partner. Wear appropriate clothing, proper footwear, and eye protection. Report all accidents and possible hazards to the teachers. Remove all unnecessary materials from the work area and completely clean up the work area after the experiment. Always make safety your first consideration in the laboratory. Safety Contract: I will: Follow all instructions given by the teacher. Protect eyes, face and hands, and body while conducting class activities. Carry out good housekeeping practices. Know where to get help fast. Know the location of the first aid and firefighting equipment. Conduct myself in a responsible manner at all times in a laboratory situation. I, _______________________, have read and agree to abide by the safety regulations as set forth above and also any additional printed instructions provided by the teacher. I further agree to follow all other written and verbal instructions given in class. Signature: ____________________________ EL8_2014 Date: ___________________ 11 Pre-Lab Safety Worksheet and Approval Form This form must be completed with the teacher’s collaboration before the lab. Student Researcher Name: __________________________________________Period # _____ Title of Experiment: ____________________________________________________________ Place a check mark in front of each true statement below: 1. I have reviewed the safety rules and guidelines. 2. This lab activity involves one or more of the following: Human subjects (Permission from participants required. Subjects must indicate willingness to participate by signing this form below.) Vertebrate Animals (requires an additional form) Potentially Hazardous Biological Agents (Microorganisms, molds, rDNA, tissues, including blood or blood products, all require an additional form.) Hazardous chemicals (such as: strong acids or bases) Hazardous devices (such as: sharp objects or electrical equipment) Potentially Hazardous Activities (such as: heating liquids or using flames) 3. I understand the possible risks and ethical considerations/concerns involved in this experiment. 4. I have completed an Experimental/Engineering Design Diagram. Show that you understand the safety and ethical concerns related to this lab by responding to the questions below. Then, sign and submit this form to your teacher before you proceed with the experiment (use back of paper, if necessary). A. Describe what you will be doing during this lab. B. What are the safety concerns with this lab that were explained by your teacher? How will you address them? C. What additional safety concerns or questions do you have? D. What ethical concerns related to this lab do you have? How will you address them? Student Researcher’s Signature/Date: Teacher Approval Signature: ____________________________________ ______________________________ Human Subjects’ Agreement to Participate: _______________________________ ____________________________ Printed Name/Signature/Date Printed Name/Signature/Date _______________________________ _____________________________ Printed Name/Signature/Date Printed Name/Signature/Date EL8_2014 12 Parts of a Lab Report A Step-by-Step Checklist A good scientist reflects on their work by writing a lab report. A lab report is a recap of what a scientist investigated. It is made up of the following parts. Title (underlined and on the top center of the page) Benchmarks Covered: Your teacher should provide this information for you. It is a summary of the main concepts that you will learn about by carrying out the experiment. Problem Statement: Identify the research question/problem and state it clearly. Variables and Control Test: Identify the variables in the experiment. State those over which you have control. There are three types of variables. 1. Test Variable (Independent Variable): (also known as the tested variable) the factor that can be changed by the investigator (the cause). 2. Outcome Variable (Dependent Variable): (also known as the outcome variable) the observable factor of an investigation which is the result or what happened when the independent variable was changed. 3. Controlled variables (Constants): the other identified independent variables in the investigation that are kept constant or remain the same during the investigation. Identify the control test. A control lest is the separate experiment that serves as the standard for comparison to identify experimental effects, changes of the dependent variable resulting from changes made to the independent variable. Potential Hypothesis (e.g.): State the hypothesis carefully. Do not just guess but try to arrive at the hypothesis logically and, if appropriate, with a calculation. Write down your prediction as to how the test variable (independent variable) will affect the outcome variable (dependent variable) using an “if” and “then” statement. o If (state the test variable) is (choose an action), then (state the outcome variable) will (choose an action). Materials: Record precise details of all equipment used o For example: a balance weighing to +/- 0.001 g, a thermometer measuring from -10 to +110oC to an accuracy of +/- 0.1oC, etc. Record precise details of any chemicals used o For example: 5 g of copper (II) sulfate pentahydrate CuSO4.5H2O(s). Procedure: Do not copy the procedures from the lab manual or handout. Summarize the procedures; be sure to include critical steps. Give accurate and concise details about the apparatus and materials used. EL8_2014 13 Data: Ensure that all data is recorded. o Pay particular attention to significant figures and make sure that all units are stated. Present your results clearly. Often it is better to use a table or a graph. o If using a graph, make sure that the graph has a title, both axis are labeled clearly, and that the correct scale is chosen to utilize most of the graph space. Record all observations. o Include color changes, solubility changes, whether heat was evolved or taken in, etc. Results: Ensure that you have used your data correctly to produce the required result in words and provide graphs. Include any other errors or uncertainties which may affect the validity of your result. Conclusion and Evaluation: A conclusion statement answers the following 7 questions in at least three paragraphs. o First Paragraph: Introduction 1. What was investigated? a. Describe the problem. 2. Was the hypothesis supported by the data? a. Compare your actual result to the expected result (either from the literature, textbook, or your hypothesis) b. Include a valid conclusion that relates to the initial problem or hypothesis. 3. What were your major findings? a. Did the findings support or not support the hypothesis as the solution to the restated problem? b. Calculate the percentage error from the expected value. o Middle Paragraphs: These paragraphs answer question 4 and discusses the major findings of the experiment using data. 4. How did your findings compare with other researchers? a. Compare your result to other students’ results in the class. The body paragraphs support the introductory paragraph by elaborating on the different pieces of information that were collected as data that either supported or did not support the original hypothesis. Each finding needs its own sentence and relates back to supporting or not supporting the hypothesis. The number of body paragraphs you have will depend on how many different types of data were collected. They will always refer back to the findings in the first paragraph. o Last Paragraph: Conclusion 5. What possible explanations can you offer for your findings? a. Evaluate your method. b. State any assumptions that were made which may affect the result. 6. What recommendations do you have for further study and for improving the experiment? a. Comment on the limitations of the method chosen. b. Suggest how the method chosen could be improved to obtain more accurate and reliable results. 7. What are some possible applications of the experiment? a. How can this experiment or the findings of this experiment be used in the real world for the benefit of society? EL8_2014 14 Parts of a Lab Report Reminder Step 1: Stating the Purpose/Problem What do you want to find out? Write a statement that describes what you want to do. It should be as specific as possible. Often, scientists read relevant information pertaining to their experiment beforehand. The purpose/problem will most likely be stated as a question such as: “What are the effects of _________ on ___________?” Step 2: Defining Variables TEST VARIABLE (TV) (also called the independent variable) – The variable that is changed on purpose for the experiment; you may have several levels of your test variable. OUTCOME VARIABLE (OV) (also called the dependent variable) – The variable that acts in response to or because of the manipulation of the test variable. CONTROLLED VARIABLES (CV) – All factors in the experiment that are NOT allowed to change throughout the entire experiment. Controlling variables is very important to assure that the results are due only to the changes in the test variable; everything (except the test variable) must be kept constant in order to provide accurate results. Step 3: Forming a Hypothesis A hypothesis is an inferring statement that can be tested. The hypothesis describes how you think the test variable will respond to the outcome variable. (i.e., If….., then……) It is based on research and is written prior to the experiment. Never change your hypothesis during the experiment. For example: If the temperature increases, then the rate of the reaction will increase. Never use “I,” “we,” or “you” in your hypothesis (i.e. I believe or I think that…) It is OK if the hypothesis is not supported by the data. A possible explanation for the unexpected results should be given in the conclusion Step 4: Designing an Experimental Procedure Select only one thing to change in each experimental group (test variable). Change a variable that will help test the hypothesis. The procedure must tell how the variable will be changed (what are you doing?). The procedure must explain how the change in the variable will be measured. The procedure should indicate how many trials would be performed (usually a minimum of 3-4 for class experiments). It must be written in a way that someone can copy your experiment, in step by step format. Step 5: Results (Data) Qualitative Data is comprised of a description of the experimental results (i.e. larger, faster….). Quantitative Data is comprised of results in numbers (i.e. 5 cm, 10.4 grams) The results of the experiment will usually be compiled into a table/chart for easy interpretation. A graph of the data (results) may be made to more easily observe trends. Step 6: Conclusion The conclusion should be written in paragraph form. It is a summary of the experiment, not a stepby-step description. Does the data support the hypothesis? If so, you state that the hypothesis is accepted. If not, you reject the hypothesis and offer an explanation for the unexpected result. You should summarize the trend in data in a concluding statement (ex: To conclude, the increase in temperature caused the rate of change to increase as shown by the above stated data.). Compare or contrast your results to those from similar experiments. You should also discuss the implications for further study. Could a variation of this experiment be used for another study? How does the experiment relate to situations outside the lab? (How could you apply it to real world situations?) EL8_2014 15 Student’s name: _____________________________________________ Date: ________________Period: _____ Experimental Design Diagram This form should be completed before experimentation. Title: Problem Statement: Null Hypothesis: Research Hypothesis: Test Variable (Independent Variable) Number of Tests: Subdivide this box to specify each variety. Control Test: # of Trials per Test: Outcome Variable (Dependent Variable) Controlled Variables 1. 2. 3. 4. 5. 6. EL8_2014 16 Experimental Design Diagram Hints: Title: A clear, scientific way to communicate what you’re changing and what you’re measuring is to state your title as, "The Effect of ____________on__________." The tested variable is written on the first line above and the outcome variable is written on the second line. Problem Statement: Use an interrogative word and end the sentence with a question mark. Begin the sentence with words such as: How many, How often, Where, Will, or What. Avoid Why. Null Hypothesis: This begins just like the alternate hypothesis. The sentence should be in If ............, then........... form. After If, you should state the TV, and after the then, you should state that there will be no significant difference in the results of each test group. Research Hypothesis: If ____________(state the conditions of the experiment), then ____________(state the predicted measurable results). Do not use pronouns (no I, you, or we) following If in your hypothesis. Test Variable (TV): This is the condition the experimenter sets up, so it is known before the experiment (I know the TV before). In middle school, there is usually only one TV. It is also called the independent variable, the IV. Number of Tests: State the number of variations of the TV and identify how they are different from one another. For example, if the TV is "Amount of Calcium Chloride" and 4 different amounts are used, there would be 4 tests. Then, specify the amount used in each test. Control Test: This is usually the experimental set up that does not use the TV. Another type of control test is one in which the experimenter decides to use the normal or usual condition as the control test to serve as a standard to compare experimental results against. The control is not counted as one of the tests of the TV. In comparison experiments there may be no control test. Number of Trials: This is the number of repetitions of one test. You will do the same number of repetitions of each variety of the TV and also the same number of repetitions of the control test. If you have 4 test groups and you repeat each test 30 times, you are doing 30 trials. Do not multiply 4 x 30 and state that there were 120 trials. Outcome Variable(s): This is the result that you observe, measure and record during the experiment. It’s also known as the dependent variable, OV. (I don’t know the measurement of the OV before doing the experiment.) You may have more than one OV. Controlled Variables or Variables Held Constant: Controlled Variables (Constants) are conditions that you keep the same way while conducting each variation (test) and the control test. All conditions must be the same in each test except for the TV in order to conclude that the TV was the cause of any differences in the results. Examples of Controlled Variables (Constants): Same experimenter, same place, time, environmental conditions, same measuring tools, and same techniques. EL8_2014 17 CONCLUSION WRITING Claim, Evidence and Reasoning Students should support their own written claims with appropriate justification. Science education should help prepare students for this complex inquiry practice where students seek and provide evidence and reasons for ideas or claims (Driver, Newton and Osborne, 2000). Engaging students in explanation and argumentation can result in numerous benefits for students. Research shows that when students develop and provide support for their claims they develop a better and stronger understanding of the content knowledge (Zohar and Nemet, 2002). When students construct explanations, they actively use the scientific principles to explain different phenomena, developing a deeper understanding of the content. Constructing explanations may also help change students’ view of science (Bell and Linn, 2000). Often students view science as a static set of facts that they need to memorize. They do not understand that scientists socially construct scientific ideas and that this science knowledge can change over time. By engaging in this inquiry practice, students can also improve their ability to justify their own written claims (McNeill et al., 2006). Remember when providing evidence to support a claim, the evidence must always be: Appropriate Accurate Sufficient The rubric below should be used when grading lab reports/conclusions to ensure that students are effectively connecting their claim to their evidence to provide logical reasons for their conclusions. Base Explanation Rubric Component 0 Claim - A conclusion that answers the original question. Evidence – Scientific data that supports the claim. The data needs to be appropriate and sufficient to support the claim. Reasoning – A justification that links the claim and evidence. It shows why the data count as evidence by using appropriate and sufficient scientific principles. Does not make a claim, or makes an inaccurate claim. Does not provide evidence, or only provides inappropriate evidence (evidence that does not support the claim). Does not provide reasoning, or only provides reasoning that does not link evidence to claim Level 1 Makes an accurate but incomplete claim. Provides appropriate but insufficient evidence to support claim. May include some inappropriate evidence. Provides reasoning that links the claim and evidence. Repeats the evidence and/or includes some – but not sufficient – scientific principles. 2 Makes an accurate and complete claim. Provides appropriate and sufficient evidence to support claim. Provides reasoning that links evidence to claim. Includes appropriate and sufficient scientific principles. McNeill, K. L. & Krajcik, J. (2008). Inquiry and scientific explanations: Helping students use evidence and reasoning. In Luft, J., Bell, R. & GessNewsome, J. (Eds.). Science as inquiry in the secondary setting. (p. 121-134). Arlington, VA: National Science Teachers Association Press. EL8_2014 18 Engineering Design Process Step 1 Identify the Need or Problem Step 8 Redesign Step 2 Research the Need or Problem Step 7 Communicate the Solution(s) Step 3 Develop Possible Solution(s) Step 6 Test and Evaluate the Solution(s) Step 5 Construct a Prototype Step 4 Select the Best Possible Solution(s) 1. Identify the need or problem 2. Research the need or problem a. Examine current state of the issue and current solutions b. Explore other options via the internet, library, interviews, etc. c. Determine design criteria 3. Develop possible solution(s) a. Brainstorm possible solutions b. Draw on mathematics and science c. Articulate the possible solutions in two and three dimensions d. Refine the possible solutions 4. Select the best possible solution(s) a. Determine which solution(s) best meet(s) the original requirements 5. Construct a prototype a. Model the selected solution(s) in two and three dimensions 6. Test and evaluate the solution(s) a. Does it work? b. Does it meet the original design constraints? 7. Communicate the solution(s) a. Make an engineering presentation that includes a discussion of how the solution(s) best meet(s) the needs of the initial problem, opportunity, or need b. Discuss societal impact and tradeoffs of the solution(s) 8. Redesign a. Overhaul the solution(s) based on information gathered during the tests and presentation Source(s): Massachusetts Department of Elementary and Secondary Education EL8_2014 19 Teacher EXPERIMENTAL DESIGN: PASTA STRENGTH Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.N.1.1 Define a problem from the 8th grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of various types: systematic observations, or experiments, identify variables. AA (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) SC.8.P.8.2 Differentiate between weight and mass, recognizing that weight is the amount of gravitational pull on an object and is distinct from, though proportional to, mass. (Also assessed as SC.6.P.13.1, SC.6.P.13.2) Purpose: Students will design an experiment that tests the strength of dry pasta. They will construct a bridge made out of one pasta noodle and textbooks. They will test the strength using pennies. An experiment is an organized series of steps used to test a hypothesis. Experimental design is a specific set of directions for designing and carrying out an experiment, so that the results are as valid as possible. Experimental design seeks to eliminate experimental error and to insure that the results are due to the factor being tested. The following vocabulary is used in experimental design: o Test Variable: The factor controlled by the experimenter. This might also be described as the change made by the experimenter on purpose. It is sometimes called the manipulated variable o Outcome Variable: The factor that changes because of what the experimenter does. The dependent variable is the change that occurs because of what the experimenter does. It is sometimes called the responding variable. o Constant Variable: The factor(s) that remain the same so that there is only one variable that is tested. o Repeated Trials: The number of times that the experiment is done. Students will recognize how the mass of a penny can test the strength of a pasta noodle as a result of the downward pull of gravity on the penny. Materials (per group): Ruler 50-100 Pennies Styrofoam/Plastic Cup and string to make a handle (see picture) 5 Strands of uncooked pasta (provide variety) Tape Procedures: Before Activity Preparation: Teacher will create the bucket cups prior to the lesson. See image below. Engage: Optional: Teacher will play Scientific Method song for students. Teacher will have two bridges made out of uncooked pasta of the same type (spaghetti, linguini, or angel hair). The first bridge will consist of 5 noodles and the second will consist of 8 noodles (amount is up to the teacher but make sure to have a difference in amount). The teacher will demonstrate how to test the strength by having a cup with a string hanging from the pasta bridge. The teacher will ask students to predict how much EL8_2014 20 Teacher mass both bridges will hold. Concepts to incorporate during discussion: The strength of the bridge is tested by applying a downward force (pennies placed in cup hanging off of noodle). The strength of the pasta bridge will depend on the physical properties of the pasta noodle (length and density). Discussion: Teacher will establish the purpose of the activity and review the scientific method and experimental design. The teacher will explain that they will test the strength of pasta, but will only be able to build a bridge out of ONE pasta noodle. The teacher will push students to think of different ways to test this question. Teacher will pass out the lab handout activity for students at this time for students to take notes and prepare for the activity. Guiding Questions – Possible answers are not limited to the ones below: 1. What factors influence the strength of pasta noodles? Factors such as length, width, and thickness influence the strength of pasta noodles. For example, thicker noodles may be stronger than thin ones. 2. How can we test the strength of pasta noodles? We can make a bridge out of a pasta noodle and test its strength by placing a mass on it or hanging something on it to see how much the pasta noodle can hold. 3. How can we manipulate the factors to test the strength of pasta noodles? We can test the different types of pasta such as spaghetti, linguini, and angel hair; We can test the different brands of one type of pasta; We can test the distance the desks are placed that the pasta bridge covers (low level) During Activity Explore: Teacher will monitor students as they design their experiment and test their hypothesis in groups of 4-5. See student handout for details on what students will be creating. Students are writing their experimental plan and will execute the experiment once the procedures are complete and data table is organized. Guiding Questions (as students design experiment): 1. What is our problem statement? Possible Problem Statements: How does the type of pasta affect the amount of mass it can hold? How does the brand of (spaghetti/linguini/angel hair) pasta affect the amount of mass it can hold? How does the distance that (spaghetti/linguini/angel hair) pasta spans affect the amount of mass the pasta can hold? 2. What is our hypothesis? Possible Hypotheses: If I create a bridge out of an uncooked linguini pasta noodle, it will hold the most amount of mass than if I were to use angel hair or spaghetti. If I use the EL8_2014 21 Teacher 3. What variables must we consider when testing the strength of the pasta noodle? Test Variable: Type of pasta, brand of pasta, and distance between desks/textbooks. Outcome Variable: Amount of pennies that the pasta bridge can hold Constant Variable: Number of pasta noodles, distance between desks/textbooks (if testing type or brand), brand of pasta (if testing type), type of pasta (if testing brand/distance). 4. How will you design the experiment? What will your procedures be? Procedures will vary depending on what students choose to test. 5. Are your procedures detailed enough that another group can replicate the process? Students should explain that every procedure is numbered and includes a verb that clearly states what they will do at each step. 6. How many trials will you conduct and why is it important to conduct multiple trials? Students should explain that they will repeat the process of taping one strand of pasta noodle and placing pennies in the bucket X amount of times, requiring X amount of noodles for their experiment. 7. How will you record the data for your experiment? Guide students to create a table with multiple trials. Explain: Teacher will monitor students as they execute their experiment, collect data, and analyze data. As students are collecting data the teacher will closely monitor how students organize their data tables in collecting information. 1. Based on your test and outcome variables, how are you going to organize your data table? 2. How will you show that your group is conducting multiple trials? After Activity Elaborate: Teacher will monitor students as they write conclusions to their experiment. Students will elaborate on their understanding of the scientific process and articulate their understanding of the results of the experiment through the Claim-Evidence-Reasoning writing process. Evaluate: Teacher will evaluate understanding of the scientific method and experimental design based on their finished lab report product. EL8_2014 22 Student LAB TITLE: ____________________________________________________ Scientific Question/Problem Statement Hypothesis Test your hypothesis Test Variable: Outcome Variable: Constant Variable(s): Procedures EL8_2014 23 Student Data Collection Conclusion Claim – Write a statement that answers the original question. Evidence – Write the scientific data that supports the claim. The data needs to be appropriate and sufficient to support the claim. Reasoning – Explain how the evidence justifies your claim. It shows why the data count as evidence by using appropriate and sufficient scientific principles. EL8_2014 24 Student “WHAT’S THE MATTER?” INQUIRY LAB Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.P.8.4. Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. SC.8.P.9.2. Differentiate between physical changes and chemical changes. SC.8.P.9.3 Investigate and describe how temperature influences chemical changes. Purpose Students will identify different classes of matter based on physical properties by separating a mixture. Students will observe and explore the properties of different substances. Students will test how different substances interact with each other Students will test how temperature influences chemical changes. Prior Knowledge: Matter is divided into the four basic states of solid, liquid, gas, and plasma. Matter is classified based on composition. Matter is identified by its characteristic physical properties. Physical properties are those that can be determined without altering the composition of the substance, such as, color, odor, density, strength, elasticity, magnetism, and solubility. Chemical properties are descriptions of the substance and its reactions with other substances to create new substances with new properties. These chemical properties are identified through chemical reactions. Evidence of a chemical reaction possibly occurring can be seen through a color change, temperature change, evolution of a gas, and the formation of a new substance. Materials (per group): Part 1 – Separating Mixture Mystery Mixture (sugar, sand, water, wood chips, and iron fillings or staples) Hot Plate Triple Beam Balance Coffee Filter Magnet Beaker Thermometer Graduated Cylinder Part 2 – Physical & Chemical Changes Test tubes Magnet Wooden Splints Thermometer Water Baking Soda Effervescent tablet Salt Magnifying glass Hot Plate Iron Fillings Graduated Cylinder Small beaker Vinegar Procedures for Teacher Before Teacher will create mystery mixture in a beaker for each lab group, which consists Activity of sugar, sand, water, wood chips, and iron (fillings or staples). Teacher will engage students through the following activities: 1. “Mystery balloons”: place common objects or materials (penny, key, battery, flour, etc.) in deflated rubber balloons and tie. Have students use their senses to try to identify the contents based on physical properties. 2. Show Study Jams-Properties of Matter. Teacher will explain that today’s lab will be done in three parts—part 1 is separating a mixture, part 2 is identifying characteristics of separated samples, and part 3 is an introduction into physical and chemical changes. EL8_2014 25 Student During Activity Teacher will pass out student hand out to begin activity and will pass out the mystery mixture. Part 1 – Separating Mixtures Teacher will ask students to examine the mystery mixture and think about how they would separate it. Teacher will ask students to create a set of procedures that can be replicated to separate the mixture. The possible steps are written in red. Students should create their OWN procedures. 1. Run magnet through mixture to separate iron based on magnetism. 2. Pour water over mixture to separate wood based on density. Wood is less dense than water. 3. Use filter to remove sand from mixture since sand is not soluble in water. 4. Use hot plate to separate sugar from water. Water will evaporate first since it has a lower boiling point than sugar. If students are having difficulty coming up with procedures, ask them to list the properties of matter (magnetism, density, particle size, and solubility) After students create procedures, pass out materials and have them execute their investigation. Teacher will monitor as students answer the following questions: 1. How did you separate the materials in the beaker? Answers will vary. 2. Why is it important for scientists to write detailed procedures? So that other scientists can replicate the study and verify the validity of the results. 3. Would the physical properties of a material change if the size of the material is changed? Explain. No, physical properties are independent of sample size. 4. Did you have to completely alter /chemically change any of the materials to measure their physical properties? Explain. No, can measure physical properties without changing the substance. Part 2 – Identifying Properties & Part 3 – Physical & Chemical Changes After students execute procedures, review with class the methods used in separating mixtures. Teacher will then tell students to move to the second part of the lab. Teacher will circulate and monitor as students answer the table. When students are done with Part 2, allow them to move on to Part 3. Students will follow procedures and collect data. Teacher will monitor as students answer the following questions: 1. How do you determine which sample is the most soluble? List the samples from highest to lowest solubility. I determined solubility by mixing the substance with water and observed how quickly and easily it dissolved. 2. How could you determine the difference between water and vinegar? Which physical properties were different between these liquids? Water and vinegar have different odors, which is a physical property that we use to help determine its identity. Important Note: Students may not know what the difference is between a physical and chemical change. This activity is to get students thinking about physical and chemical changes for the next topic. EL8_2014 26 Student After Activity After students have completed the lab procedures they are to answer their conclusion questions: How did you determine whether you thought the mixture was physical or a chemical change? Explain your reasoning. Answers may vary because students may not know explicitly the difference between a physical and chemical change. Ideally, they would explain that physical changes only change its shape or size without changing the molecules or chemical composition of the object. Chemical changes create new substances or cannot be turned back to what is original was. Scientists often find mysterious materials. Explain how physical properties are important for identifying unknown substances. Scientists can use the various physical properties such as melting point, boiling point, thermal or electrical conductivity, magnetism, density and solubility of the unknown substance to compare to known substances and correctly identify the substance or discover a new substance. EL8_2014 Evaluate student understanding of objectives through conclusion writing and/or answers to aligned test questions. 27 Student Lab Activity: __________________________________________ Benchmarks: SC.8.P.8.4. Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. SC.8.P.9.2. Differentiate between physical changes and chemical changes. SC.8.P.9.3 Investigate and describe how temperature influences chemical changes. Part 1: Separating Matter Purpose: You will design your own method to separate the mystery mixture based on physical properties of each substance. Observations: 1. What substances do you think are in the mystery mixture? Explain your reasoning. 2. What are physical properties that we use to identify substances? Scientific Question: Procedures: EL8_2014 28 Student Material Separating Matter Data Table 1 Physical Property used to Explanation separate from mixture Sugar Sand Wood Iron Analysis Questions 1. How did you separate the materials in the beaker? 2. Why is it important for scientists to write detailed procedures? 3. Would the physical properties of a material change if the size of the material is changed? Explain. 4. Did you have to completely alter /chemically change any of the materials to measure their physical properties? Explain. EL8_2014 29 Student Part 2: Identifying Physical Properties of Matter Procedures 1. Examine each sample in the test tube and record your observations in Table 1. 2. Use a magnifying glass if necessary to describe the particle size as small, medium, large, crystal-structure, etc. Be as descriptive as you can. 3. Use the magnet to test each sample for magnetic properties. 4. Test the solubility of the solids by taking half of the sample and mixing it in a new test tube that has 5 mL of water. Use a wooden splint to mix the substance with the water and record observations. Data Identifying Physical Properties Data Table 2 Color Odor Particle Size Magnetic? Sample Soluble? State of Matter Water N/A Vinegar N/A Salt Baking Soda Iron fillings Effervescent Tablet 1. How do you determine which sample is the most soluble? List the samples from highest to lowest solubility. 2. How could you determine the difference between water and vinegar? Which physical properties were different between these liquids? 1. 2. 3. 4. Part 3: Physical and Chemical Changes Mix water with salt and record your observations in Table 2. Mix the iron fillings with hydrogen peroxide and record observations in Table 2. Mix the hydrogen peroxide with water and record observations in Table 2. Mix vinegar with baking soda and record observations in Table 2. Mixture Table 2 Observations Physical or Chemical Change? Water and Salt Water and Effervescent Tablet Vinegar and Baking Soda EL8_2014 30 Student Conclusion Questions How did you determine whether you thought the mixture was physical or a chemical change? Explain your reasoning. Scientists often find mysterious materials. Explain how physical properties are important for identifying unknown substances. Claim – Write a statement that answers the original question. Evidence – Write the scientific data that supports the claim. The data needs to be appropriate and sufficient to support the claim. Reasoning – Explain how the evidence justifies your claim. It shows why the data count as evidence by using appropriate and sufficient scientific principles. EL8_2014 31 Teacher PHYSICAL CHANGES & CHEMICAL CHANGES IN MATTER Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.P.9.2 Differentiate between physical changes and chemical changes. (AA) (Also assesses SC.8.P.9.1 and SC.8.P.9.3.) SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. Purpose: Students will differentiate between physical changes and chemical changes by mixing a variety of substances in test tubes with red cabbage juice. Important Notes: There are two versions of this lab with separate directions for each outlined in the “Procedure” table. The use of vinegar and calcium chloride will need to be accompanied by the use of a ventilation fan in case of nasal sensitivity, allergy issues, or asthma. Be sure to read precautions on the calcium chloride container. Calcium Chloride can burn the skin. Students should use gloves when handling this substance. If you prepare small cups with quantities for each set of students you may want to cover the cups to prevent inhalation issues. Guiding Questions: How does changing what you add to each substance affect it? Answers may vary. How could you explain the similarities and differences between what you see before you start your investigation and after you have completed your tests? Answers may vary. What is a physical change? Any change that changes a substance’s shape, texture, or other physical property without altering its chemical composition. What is a chemical change? Any change that alters the chemical composition of a substance. How can you tell something has stayed the same or changed into something new? A substance has undergone a chemical change when a gas is released, a precipitate has formed, an odor is released, or when its color changes (although sometimes color changes don’t always necessarily mean a chemical change occurred). Materials (per group) Beakers (2) Test tubes (6) Test tube rack Thermometer Stirrers Water Milk Vinegar Cabbage Juice Baking Soda Calcium Chloride Procedure Option A (Teacher Directed) Before Preparation Teacher will prepare test tubes, all of which contain purple cabbage juice, about 5-10 ml depending on the size of test tubes. Engage Teacher may demonstrate different changes (both physical and chemical) in front of students without telling what is happening. Teacher may also play videos of physical and chemical changes that occur in matter. EL8_2014 32 Teacher During Explore Teacher will direct to students to work together in pairs for this option. Teacher will explain that students will have 5 test tubes filled with cabbage juice to test materials for reactions. Teacher will list what each test tube is to test. Students will write their predictions as to what they think will happen. Students will test 5 liquids/materials with the cabbage juice: o Test tube 1: water (5 ml) o Test tube 2: vinegar (5 ml) o Test tube 3: baking soda (a pinch or ¼ a small spoonful) o Test tube 4: calcium carbonate (¼ a small spoonful) o Test tube 5: milk (5 ml) Teacher will instruct students on how to mix materials and how to take the temperature of each test tube before and during the reaction. Be sure students clean the thermometer between each reaction to avoid cross reactions. Students will write down their observations. Explain The teacher will write vocabulary on the board and ask students to use these terms during their discussions: Substance Temperature Change of State Mixture Solution Property Solid Liquid Gas After The teacher will facilitate student discussions of the Guiding Questions. Elaborate The teacher will give a demonstration at the end of the activity that involves mixing vinegar, purple cabbage juice, milk, baking soda, and calcium chloride. Students will make predictions, discuss, and explain the physical and/or chemical changes they think are involved (Predict/Observe/Explain). You may also want to talk about how purple cabbage juice is also used to tell whether or not something is an acid or a base, and tell students it is something they will also be learning about. When the cabbage juice changes color, it is a chemical change resulting in either blue (bases) or red (acids). Evaluate Students will write a Claim-Evidence-Reasoning Conclusion to the lab activity using evidence to support their reasoning as to whether a chemical or physical change occurred in each combination. Procedure Option B (Inquiry) Before Preparation Teacher will set up test tubes on test tube rack. Teacher will set up a tray of all materials/liquids for students to choose from, EL8_2014 33 Teacher but will not place in test tubes as to allow students to create their own combinations. Engage Teacher may demonstrate different changes (both physical and chemical) in front of students without telling what is happening. Teacher may also play videos of physical and chemical changes that occur in matter. During Explore Teacher will direct to students to work in groups of 2 or 3 to design an experiment to test each substance. Teacher will direct students to write out their procedures and include a table that organizes their data and shows each liquid being tested both with the other liquids and with the two solids. The table should also include space for documenting observations before and after testing each substance. Teacher will instruct students on how to mix materials and how to take the temperature of each test tube before and during the reaction. Be sure students clean the thermometer between each reaction to avoid cross reactions. Students will write down their observations. Explain The teacher will write vocabulary on the board and ask students to use these terms during their discussions: Substance Temperature Change of State Mixture Solution Property Solid Liquid Gas After The teacher will facilitate student discussions of the Guiding Questions. Elaborate The teacher will give a demonstration at the end of the activity that involves mixing vinegar, purple cabbage juice, milk, baking soda, and calcium chloride. Students will make predictions, discuss, and explain the physical and/or chemical changes they think are involved (Predict/Observe/Explain). You may also want to talk about how purple cabbage juice is also used to tell whether or not something is an acid or a base, and tell students it is something they will also be learning about. When the cabbage juice changes color, it is a chemical change resulting in either blue (bases) or red (acids). Evaluate Students will write a Claim-Evidence-Reasoning Conclusion to the lab activity using evidence to support their reasoning as to whether a chemical or physical change occurred in each combination. EL8_2014 34 Student Student – OPTION A Lab Activity: __________________________________________ Benchmarks: SC.8.P.9.2. Differentiate between physical changes and chemical changes. SC.8.P.8.4. Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. SC.8.P.9.3 Investigate and describe how temperature influences chemical changes. Purpose: You will design your experiment to test the reactions of different liquids and solids to differentiate between physical changes and chemical changes. Prediction: Predict whether you think a physical change or a chemical change will occur when each of the following substances is mixed with red cabbage juice. Substance 1 Water 2 Vinegar 3 Baking Soda 4 Calcium Carbonate 5 Milk Physical or Chemical Change? Procedures: 1. Gather materials and safety equipment. Label test tubes with numbers 1-5. All test tubes have red cabbage juice. 2. Take the temperature of the cabbage juice in each test tube and record in table. 3. Pour 5mL of water into test tube 1 and record the temperature and any changes you observe. 4. Repeat step #3 for 5mL vinegar in test tube 2, a pinch of baking soda in test tube 3, ¼ spoonful of calcium carbonate in test tube 4, and 5mL of milk in test tube 5. Observation Table: Substance 1 Water 2 Vinegar 3 Baking Soda 4 Calcium Carbonate 5 Milk EL8_2014 Record Observations Physical or Chemical Change? Temp. Before Temp. After 35 Student Reflection Questions: 1. How could you explain the similarities and differences between what you see before you start your investigation and after you have completed your tests? 2. What is a physical change? 3. What is a chemical change? 4. How can you tell something has stayed the same or changed into something new? Conclusion: Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 36 Student Student – OPTION B Inquiry Lab Activity: __________________________________________ Benchmarks: SC.8.P.9.2. Differentiate between physical changes and chemical changes. SC.8.P.8.4. Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. SC.8.P.9.3 Investigate and describe how temperature influences chemical changes. Purpose: You will design your experiment to test the reactions of different liquids and solids to differentiate between physical changes and chemical changes. Preparation: List the substances your group will combine and write down your prediction in the table below. Substance 1 Substance 2 Physical or Chemical Change? Procedures: EL8_2014 37 Student Observation Table: Reflection Questions: 1. How does changing what you add to each substance affect it? 2. How could you explain the similarities and differences between what you see before you start your investigation and after you have completed your tests? 3. What is a physical change? 4. What is a chemical change? 5. How can you tell something has stayed the same or changed into something new? Conclusion: How can you differentiate between a physical and chemical change? Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 38 Teacher ATOMIC MODELS Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.P.8.1 Explore the scientific theory of atoms (also known as atomic theory) by using models to explain the motion of particles in solids, liquids, and gases. Assessed as SC.8.P.8.5 (Cognitive Complexity: Level 2: Basic Application of Skills & Concepts) SC.8.P.8.7 Explore the scientific theory of atoms (also known as atomic theory) by recognizing that atoms are the smallest unit of an element and are composed of sub-atomic particles (electrons surrounding a nucleus containing protons and neutrons). Assessed as SC.8.P.8.5 (Cognitive Complexity: Level 2: Basic Application of Skills & Concepts) Purpose: Students will explain that atoms are the smallest unit of an element and are composed of subatomic particles by drawing and/or creating models of an atom. Students will describe size and charge of the subatomic particles proton, neutron, and electron. Guiding Questions: What is the smallest unit of matter? How does the structure of an atom compose all matter? Materials Handout & Periodic Table of Elements Procedure Before During EL8_2014 Preparation Teacher will set up projector to illustrate atoms in a pencil. Teacher will have handouts of student “Atomic Models” worksheet. Optional: Periodic Table for Elaborate activity. Engage Ask students to cut a piece of paper as small as they can. Explain to students that the smallest unit of matter is called an “atom” and is smaller than the piece of paper they cut and cannot be seen by the human eye. Explore Show a picture of a pencil point and how the carbon atoms look at the molecular level. Project the image Pencil Zoom. Ask students questions: o What are the three different tiny particles that make up an atom? Protons, neutrons, and electrons. o Which of these is in the center of the atom? Protons and neutrons are in the center (nucleus) of the atom. You may want to mention that hydrogen is the only atom that usually has no neutrons. The nucleus of most hydrogen atoms is composed of just 1 proton. A small percentage of hydrogen atoms have 1 or even 2 neutrons. o What zooms around the nucleus of an atom? Electrons o What are the charges of these particles? Proton—positive; electron—negative; neutron—no charge. The charge on the proton and electron are exactly the same size but opposite. The 39 Teacher After EL8_2014 same number of protons and electrons exactly cancel one another in a neutral atom. Teacher will draw the current model of the atom and students will follow along. Students will then create their own atomic models in their handout. Explain Students will make the connection between atoms and matter through drawings and explanations in their handout. Teacher will circulate the classroom providing assistance to students with misconceptions. Elaborate Students will be given a periodic table to read and look for other elements that they have not created atomic models for to create their own examples of how an elements’ atoms combine to form a piece of matter. Evaluate Teacher will evaluate student understanding of objectives based on the ClaimEvidence-Reasoning conclusion that asks, “Can the atomic model explain patterns in nature?” 40 Student ATOMIC MODELS Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.P.8.1 Explore the scientific theory of atoms (also known as atomic theory) by using models to explain the motion of particles in solids, liquids, and gases. Assessed as SC.8.P.8.5 (Cognitive Complexity: Level 2: Basic Application of Skills & Concepts) SC.8.P.8.7 Explore the scientific theory of atoms (also known as atomic theory) by recognizing that atoms are the smallest unit of an element and are composed of sub-atomic particles (electrons surrounding a nucleus containing protons and neutrons). Assessed as SC.8.P.8.5 (Cognitive Complexity: Level 2: Basic Application of Skills & Concepts) Purpose: You will explain the composition of matter by illustrating various atomic models of different elements. Observation: Based on the picture below, explain the relationship between all matter and atoms. EL8_2014 41 Student Atomic Models: Matter is made up of different elements such as carbon, oxygen, magnesium, potassium, and helium. Below are everyday objects composed of elements. Draw the atomic model for the element in the table. Be sure to include the nucleus, proton, neutron, and electron. Object Element Helium Protons: 2 Neutrons: 2 Electrons: 2 Atomic Model Lithium Protons: 3 Neutrons: 3 Electrons: 3 Beryllium Protons: 4 Neutrons: 4 Electrons: 4 Boron Protons: 5 Neutrons: 5 Electrons: 5 Carbon Protons: 6 Neutrons: 6 Electrons: 6 EL8_2014 42 Student Object Element Fluorine Protons: 9 Neutrons: 9 Electrons: 9 Atomic Model Potassium Protons: 19 Neutrons: 19 Electrons: 19 Elaborate: Review the Periodic Table of Elements and look for an element that you have heard of before and draw the object that contains that element and the atomic model for that element on a separate piece of paper. Conclusion: What patterns emerge from the atomic model? Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 43 Teacher PERIODIC TABLE OF ELEMENTS Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.P.8.6 Recognize that elements are grouped in the periodic table according to similarities of their properties. Assessed as SC.8.P.8.5 (Cognitive Complexity: Level 1:Recall) Purpose: Students will be introduced to the basic information given for the elements in most periodic tables: the name, symbol, atomic number, and atomic mass for each element. Students will focus on the first 20 elements to create an imaginary periodic table that is modeled off of the Periodic Table of Elements commonly used. Students will identify trends in the periodic table by explaining that elements in the same groups have similar properties. Guiding Questions: How do we organize what we know about matter, elements, and atoms? What is the Periodic Table and how is it useful? What trends do we see in the Periodic Table? Materials Handout, Periodic Table of Elements, and Textbook Procedure Before During After EL8_2014 Preparation Print out student handouts, periodic table, and project periodic table on the board. Engage Project the image of the Periodic Table to students and explain how to read the Periodic Table Explore Students will work in groups of 2-3 to read through the “Universal Periodic Table” clues. Students will fill in their periodic table based on the clues, which require them to understand how the periodic table is organized. Explain Students will defend how they filled in their periodic table by writing their explanation of how elements are organized on the periodic table. Elaborate Students will give examples of families of elements that have common characteristics. Evaluate Teacher will evaluate student understanding of objective based on written conclusion in C-E-R that answers the question, “Is there a way to arrange the elements differently than how they organized in the Periodic Table?” Students should be able to explain how elements are arranged (increasing in order of atomic number; elements with similar characteristics are grouped in families). 44 Teacher EL8_2014 45 Teacher PERIODIC TABLE OF ELEMENTS Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.P.8.6 Recognize that elements are grouped in the periodic table according to similarities of their properties. Assessed as SC.8.P.8.5 (Cognitive Complexity: Level 1:Recall) Purpose: You have been chosen to assist a group of alien scientists. In order to be able to converse scientifically, you must learn their language, and most importantly, you must arrange their elements according to the trends that exist in the periodic table. Below are clues for the alien's elements. So far, the aliens have only discovered elements in groups 1, 2, and 13-18, and periods 1-5. Although the names of the elements are different, they must correspond to our elements if our belief of universal elements holds true. The diagram and information below will help you match your clues to the Human periodic table. Procedure: Read each clue carefully, and then place the symbol for that clue's element in the blank periodic table provided. 1. Livium (Lv): This element is responsible for life. It has 6 electrons. 2. Computerchipium (Cc): This element is important for computers. It has 14 protons. 3. Lightium (L): This is the lightest of elements; aliens used it in their aircraft until their aircraft caught fire in a horrific accident. It also has a low melting point. 4. Breathium(Br): When combined with Lightium (L), it makes the alien's most common liquid whose formula is L2 Br. It has 8 electrons. 5. Francium (F): A metal found in period 4 group 13. 6. Moonium (Mo): An element with an atomic number of 34. 7. Explodium (Ex): This element is the most reactive metal on the alien's table. It has 37 protons. 8. Violetium(V): This element is found in bananas. When burned, it has a violet colored flame. 9. Sparkium (Sp) and Burnium (Bu) are members of the alkali metal group, along with Violetium(V) and Explodium (Ex). Their reactivity, from least to greatest, is Sp, Bu, V, Ex. 10. Balloonium (Ba): A noble gas used to fill balloons. It has 2 protons. 11. Toothium (To): This element helps build strong bones and teeth. It has 20 protons. 12. Metalloidium (M) and Poisonium (Po): Two metalloids found in period 4. Po has 33 protons. 13. Lowigium (Lo): This element is a halogen found in period 4 and has 35 protons. 14. Darkbluium(Dk): Has an atomic mass of 115. 15. Hugium (Hu): This element is a noble gas on the alien's periodic table that has the most mass (131). 16. Glucinium (Gl): The element found in period 2, group 2 with a mass of 24. 17. Reactinium (Re): The most reactive non-metal on the periodic table with 9 electrons. 18. Balloonium (Ba), Signium(Si), Stableium(Sb), Supermanium (Sm), and Hugium (Hu) are all noble gases. They are arranged above from least to most massive. Ba has 2 protons. 19. Cannium (Cn): This element is used to can foods. It has 50 protons. EL8_2014 46 Teacher 20. Burnium (Bu), Blue-whitium (Bw), Bauxitium (Xi), Computerchipsium (Cc), Bringer-of-lightium (Bl), Stinkium (Sk), Purium (P), and Stableium (Sb) are all found in period 3. 21. Scottishium (Sc): An alkaline metal that is hard and tough, much like To, Bw, and Gl. It has 38 protons. 22. Infectium (If): A halogen, like Re, P, and Lo with 53 protons. 23. Abundantcium(Ab): One of the most abundant gasses in the universe. It has 7 protons, 7 neutrons, and 7 electrons. 24. Some additional clues: The number after the symbol indicates the number of protons in the nucleus of the atom: Notalonium(Na): 51, Earthium (E): 52, Boracium (B): 10. Universal Periodic Table Analysis (Use the Standard Periodic Table, not the one above): 1. What trends do you notice as elements are listed from left to right? 2. Based on the periodic table why is H, Li, Na, K, and Rb in the same column/group/family? 3. Based on the periodic table why is Be, Mg, Ca, and Sr in the same column/group/family? EL8_2014 47 Teacher 4. Based on the periodic table why is Hw, Nw, Ar, Kr, and Xe in the same colum/group/family? Conclusion: Based on this activity, is there a way to arrange the elements differently than how they organized in the Periodic Table? Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 48 Teacher Clay Elements, Molecules, and Compounds SC.8.P.8.5 Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter. (AA) SC.8.P.8.9 Distinguish among mixtures (including solutions) and pure substances (Assessed as SC.8.P.8.5) Objectives: Students will model how elements combine in a multitude of ways to produce compounds that make up all living and nonliving things. Students will differentiate among pure substances, mixtures and solutions. Essential Question: Explain how atoms of elements form molecules, compounds and mixtures which are used in your daily lives. Background Information for the Teacher: This activity is used for students to gain an understanding that atoms of elements combine to form molecules and compounds. Since students can’t see atoms, molecules and compounds, they will create models of them using different colors of clay pieces to represent the different elements. Students should understand that some molecules are elements not compounds since they are only made up of only one type of element such as hydrogen gas. Mixtures consist of different types of elements and/or compounds that are physically blended but not chemically bonded together. When students complete this activity, they should be able to differentiate between elements, compounds and mixtures. Preparation: Before Activity Preparation Before the activity, prepare the clay pieces which represent the different elements. Each group will need a bag which contains six different colors of clay pieces. Each bag should contain the number of pieces for each color that are found on the color key card. Using small bags for each color works best so that way the different colors of clay pieces don’t stick together. Engage: Show Study Jams Video: Elements and Compounds Study Jams Video: Mixtures Show examples of elements, compounds and mixtures such as sample of salt, copper, saltwater, sand and water and beaker of air. The class should have a brief discussion about the video and the samples shown During Activity Explore: Students will complete the activity: Clay Elements, Molecules and Compounds Guiding Questions: 1. What is an atom and what part of the model represents an atom? EL8_2014 49 Teacher 2. How do atoms form molecules and compounds? 3. What is the difference between molecules, compounds and mixtures? During this activity, the teacher should walk around to ensure that students understand that the atoms (clay pieces) combine to form different types of molecules, compounds and mixtures. Idea: have each group save one of their models(teacher assigns) to share during the discussion. Explain: Students will participate in a class discussion by sharing their answers to questions completed during activity and models that they created for a particular element, compound or mixture. The teacher should revisit the guiding questions to ensure that students don’t have misconceptions and have mastered the material. After Activity EL8_2014 Elaborate: Students will research and identify elements, molecules, compounds and mixtures that they use in their daily lives. They will create a drawing which represents a model of the element, molecule, compound or mixture and explain how they use each one in their daily lives. Evaluate: Teacher will evaluate student understanding of objectives based on the ClaimEvidence-Reasoning conclusion for the essential questions: Explain how atoms of elements form molecules, compounds and mixtures which are used in your daily lives. 50 Teacher Clay Model Compounds ---Color Key Count the number of clay pieces you have for each color and match to the key. Use a crayon or colored pencil to color each clay piece. Match the colors to the numbers! 8-Hydrogen 2-Sodium Nitrogen 3-Chlorine 4-Carbon 10-Oxygen 2- Clay Model Compounds ---Color Key Count the number of clay pieces you have for each color and match to the key. Use a crayon or colored pencil to color each clay piece. Match the colors to the numbers! 8-Hydrogen 2-Sodium Nitrogen 3-Chlorine 4-Carbon 10-Oxygen 2- Names: ______________________________________ Names: ______________________________________ Clay Model Compounds ---Color Key Count the number of clay pieces you have for each color and match to the key. Use a crayon or colored pencil to color each clay piece. Match the colors to the numbers! Clay Model Compounds ---Color Key Count the number of clay pieces you have for each color and match to the key. Use a crayon or colored pencil to color each clay piece. Match the colors to the numbers! 8-Hydrogen 2-Sodium Nitrogen 3-Chlorine 4-Carbon 10-Oxygen 2- 8-Hydrogen 3-Chlorine 10-Oxygen 2-Sodium 4-Carbon 2-Nitrogen Names: ______________________________________ Names: ______________________________________ Clay Model Compounds ---Color Key Count the number of clay pieces you have for each color and match to the key. Use a crayon or colored pencil to color each clay piece. Match the colors to the numbers! 8-Hydrogen 2-Sodium Nitrogen 3-Chlorine 4-Carbon 10-Oxygen 2- Clay Model Compounds ---Color Key Count the number of clay pieces you have for each color and match to the key. Use a crayon or colored pencil to color each clay piece. Match the colors to the numbers! 8-Hydrogen 3-Chlorine 10-Oxygen 2-Sodium 4-Carbon 2Nitrogen Names: ______________________________________ Names: ______________________________________ EL8_2014 51 Student STUDENT PAGE Clay Elements, Molecules, and Compounds SC.8.P.8.5 Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter. (AA) SC.8.P.8.9 Distinguish among mixtures (including solutions) and pure substances (Assessed as SC.8.P.8.5) Objectives: Students will model how elements combine in a multitude of ways to produce compounds that make up all living and nonliving things. Students will differentiate among pure substances, mixtures and solutions. Essential Question: Explain how atoms of elements form molecules, compounds and mixtures which are used in your daily lives. Atoms - small particles that make up elements and compounds Molecules - two or more atoms bonded together Elements - molecules with only one type of atom Compounds - two or more different types of atoms bonded together Mixtures – when two or more substances are physically blended but not chemically bonded together Materials: Paper Towel Toothpicks Modeling Clay Colored pencils Procedure: 1. Color the modeling clay key according to the samples of clay provided. 2. For each molecule/compound listed in the table you will need to: A. List the names of the atoms involved B. Identify the number of each atom in the molecule. C. Make the clay model D. Color the model in the table and label the name of each atom. E. Identify model as an element, compound or mixture. (You need to take apart some models to make other models. But make sure you have received the teacher's initials next to the model before you take it apart. For instance, you need to make CH4 and CO2 with the same carbon molecule.) 3. After you have completed all of the models, you must answer the questions to ensure comprehension of the material. EL8_2014 52 Student SUBSTANCE FORMULA Hydrogen Gas H2 Salt (Sodium Chloride) NaCl Methane CH4 Carbon Dioxide CO2 Oxygen Gas N2, O2, H2O, CO2 Water H2O Hydrochloric Acid HCl EL8_2014 # OF ATOMS ELEMENTS, COMPOUNDS OR MIXTURES O2 Air Sodium Hydroxide (lye) ATOM NAMES MOLECULAR MODEL Make the clay compound model and color the diagram NaOH 53 Student Carbonated Water H2O CO2 Post-Lab Questions: 1. What particle makes up all substances? 2. Which is larger, an atom or a molecule? Explain. 3. How is a compound different from a molecule? 4. Are all molecules compounds? Explain. 5. One of the properties of a pure substance it that they always exist in fixed proportions. How many hydrogen atoms are needed to form five water molecules? _______ How many oxygen atoms are needed to form five water molecules? ________ 6. Answer the Essential Question: Explain how atoms of elements form molecules, compounds and mixtures. CHALLENGE: Draw a Bohr model of an oxygen atom in first box and a hydrogen atom in the second. 7. Look at the models above. Explain why two hydrogen atoms bond to an oxygen atom to make a stable water molecule? (Hint: Look at the valence electrons) Staple your colored Clay Model Key to the front of this page EL8_2014 54 Teacher Investigating the Effect of Light Intensity on Photosynthesis Adapted from: State Adopted – Prentice Hall (Laboratory Manual B) Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.L.18.1 Describe and investigate the process of photosynthesis, such as the roles of light, carbon dioxide, water and chlorophyll; production of food; release of oxygen (Assessed as SC.8.L.18.4) Fair Game Benchmark: SC.7.E.6.6 Identify the impact that humans have had on Earth, such as deforestation, urbanization, desertification, erosion, air and water quality, changing the flow of water. (Assessed as SC.7.E.6.2) Objective/Purpose: 1. To observe how light affects photosynthesis. 2. To understand how photosynthesis in important to life. 3. To understand the consequences of what can happen when plants and trees are destroyed. Background Information: Photosynthesis is the process by which plants take carbon dioxide from the atmosphere, add water, and use the energy of sunlight to produce sugar. Photosynthesis occurs in the chloroplast, an organelle in plant cells that contains the molecule chlorophyll. Chlorophyll absorbs the energy of sunlight. That light energy is converted to chemical energy through the steps of photosynthesis. In order to carry out photosynthesis, a plant must have light. But how much light? Some plants need a lot of light. Others seem to thrive in shade. Does more light lead to more photosynthesis? In this investigation, you will examine how the intensity of light affects photosynthesis. You will also analyze the importance of photosynthesis and its need for our environment to survive. Problem Statement / Engagement: “How does the destruction of forests affect the rate of photosynthesis?” “How does this change our living environment and what consequences can a low level of photosynthesis cause to our atmosphere?” Read the entire investigation. Then, work with a partner to answer the following questions. 1. What are the products of photosynthesis? Which of these products is released from leaves as a gas? 2. What can you tell about photosynthesis if a leaf begins to produce more gas bubbles? Fewer gas bubbles? 3. What are the manipulated and responding variables in this experiment? Identify one controlled variable. Materials: Test tube Sodium bicarbonate solution 400-mL beaker Freshly cut sprig of an evergreen (such as yew) or elodea Forceps EL8_2014 Source of bright light Watch or clock with second indicator Plastic gloves Hand lens 55 Teacher Procedure: 1. Working with a partner, completely fill a test tube and a beaker with a sodium bicarbonate solution. Sodium bicarbonate will provide a source of carbon dioxide. 2. Using forceps, place a sprig of evergreen about halfway down in the test tube. Be sure that the cut end of the sprig points downward in the test tube. 3. Cover the mouth of the test tube with your thumb and turn the test tube upside down. Try not to trap any air bubbles in the test tube. EL8_2014 56 Teacher 4. Place the mouth of the test tube under the surface of the sodium bicarbonate solution in the beaker. Remove your thumb from the mouth of the test tube. 5. Gently lower the test tube inside the beaker so that the test tube leans against the side of the beaker. 6. Put the beaker in a place where it will receive normal room light. Using a hand lens, count the number of bubbles produced by the sprig in the test tube for 5 minutes. Record the number of bubbles on the Data Table below for each minute. 7. Darken the room and count the number of bubbles produced again for 5 minutes. Record the number on the Data Table for each minute. 8. Turn up the lights in the room and shine a bright light on the sprig. Count the number of bubbles produced in 5 minutes. Record the number on the Data Table for each minute. 9. Calculate the average for each light intensity. EL8_2014 57 Teacher Data (Tables and Observations): Light 1 min 2 min Intensity Room light Dim light Bright light 3 min 4 min 5 min Average Data Analysis (Calculations): 1. Observing: From what part of the sprig (stem or needle leaves) did the bubbles emerge? 2. Observing: When was the greatest number of bubbles produced? 3. Expository: Explain the data produced in the experiment in relation to the levels of photosynthesis. Results and Conclusions: 1. Drawing Conclusions: How does the intensity of light affect the rate of photosynthesis? Was your hypothesis correct or not? Explain what occurred. 2. Comparing and Contrasting: How do your results compare with those of your classmates? Are they similar? Different? How can you account for any differences in the numbers of bubbles produced? Can you identify any trends even if the actual numbers differ? 3. Closure Activity: Have the students make a Microsoft Power Point presentation about the importance of plants to our atmosphere, community and future. Have them include measures that they would implement to save our forest and stop global warming. Extension: Perform the activity again using different colors of light. What effect does each color have on the rate of photosynthesis? Notes for Teacher: Provide sprigs that are as freshly cut as possible. For better results, cut stems underwater and keep the cut ends in water until use. Prepare a saturated solution of 7 g sodium bicarbonate per 100 ml water. Pour off the solution, leaving any undissolved solid behind. EL8_2014 58 Student PHOTOSYNTHESIS LAB Problem Statement:________________________________________________________________ _________________________________________________________________________________ Hypothesis: ______________________________________________________________________ _________________________________________________________________________________ Data: Light Intensity Room light 1 min 2 min 3 min 4 min 5 min Average Dim light Bright light Observations: 1. What are the bubbles? Explain why bubbles happen. 2. Did the number of bubbles change when the light intensity was reduced? Explain why this would occur. 3. Why was the test tube placed in a beaker of water? 4. If the plant is given more CO2 what will happen to the amount of oxygen it releases? Why? 5. In this experiment, why is it important to perform multiple trials? EL8_2014 59 Student Conclusion: Based on this activity, is light important for plants to perform the process of photosynthesis? Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 60 Teacher CONSERVATION OF MASS Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.L.18.4: Cite evidence that living systems follow the Laws of Conservation of Mass and Energy. (AA) SC.8.P.8.5 Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter. (AA) (Also assesses SC.8.P.8.1, SC.8.P.8.6, SC.8.P.8.7, SC.8.P.8.8, and SC.8.P.8.9.) SC.8.P.9.2 Differentiate between physical changes and chemical changes. (AA) (Also assesses SC.8.P.9.1 and SC.8.P.9.3.) Background information: Matter and Energy Transformations The “Law of Conservation of Mass” states that when matter goes through a physical or chemical change, the amount of matter stays the same before and after the changes occur. In other words, matter cannot be created or destroyed. Engage: Teacher burns a small piece of paper inside of a beaker. Teacher asks students: “What happened to the paper?; Is there the same amount of matter in the beaker before and after?; Where did the matter go?; How can you tell?; What type of change did you observe: physical or chemical?”. Materials: Graduated Cylinder Erlenmeyer Flask Balloon Baking Soda Triple Beam Balance Spoon Answer Key: 1. Name the reactants: Baking Soda and Vinegar 2. Name the products: Sodium Acetate, Water, and Carbon Dioxide 3. Name the gas produced: Carbon Dioxide 4. Compare the mass of the closed system before and after the reaction. Explain your results. (The mass of the closed system before and after the reaction were the same because matter cannot be create nor destroyed 5. Were any new elements introduced into the closed system? Where did the gas come from? Explain. NO. The law of conservation of mass states that in any chemical reaction, matter is neither created nor destroyed. Therefore, in a balanced chemical equation you must have the same number of atoms of each element on either side of the equation. The gas came from the baking soda and vinegar 6. What evidence did you observe to indicate that a chemical reaction took place? (Bubbles indicated that a chemical reaction took place, also a new substance was form and gas was given off which inflated the balloon) 7. After the gas was released, what happened to the mass of the system and why? (The mass of the system decreased because the system was no longer closed. Some matter escaped (the gas) which caused the mass to decreased 8. Did your results support this statement? Why/Why Not? EL8_2014 61 Student CONSERVATION OF MASS SC.8.L.18.4: Cite evidence that living systems follow the Laws of Conservation of Mass and Energy. (AA) SC.8.P.8.5 Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter. (AA) (Also assesses SC.8.P.8.1, SC.8.P.8.6, SC.8.P.8.7, SC.8.P.8.8, and SC.8.P.8.9.) SC.8.P.9.2 Differentiate between physical changes and chemical changes. (AA) (Also assesses SC.8.P.9.1 and SC.8.P.9.3.) Purpose: You will test the law of conservation of mass by creating a reaction of chemicals and measuring the mass before and after of the reaction. Problem Statement Hypothesis Materials Graduated Cylinder Erlenmeyer Flask Balloon Baking Soda Triple Beam Balance Spoon Procedure - Part 1: 1. Using your graduated cylinder, measure 50 mL of vinegar. 2. Add the vinegar to your 125 mL Erlenmeyer flask. 3. Stretch your balloon out for about a minute so that it will inflate easily. 4. Using the white plastic spoon, add 10 grams of baking soda to your balloon. Use the paper funnel to avoid spilling. 5. While keeping all the baking soda in the balloon, carefully place the mouth of the balloon over the opening of the Erlenmeyer flask to make a tight seal. The balloon will hang to the side of the flask. Record/draw observations. 6. Using your Triple Beam Balance (TBB), find the mass of the closed system. (Flask, vinegar, balloon, and baking soda) Record the mass in the data table. 7. With the balloon still attached to the flask, firmly hold where the balloon is attached to the flask and lift the balloon so that the baking soda falls into the flask and combines with the vinegar. Swirl gently. 8. Record/draw all observations. EL8_2014 62 Student Observations: (diagram your observations) Before Mass of System Start (g) EL8_2014 Mass of System End (g) After Mass of System Gas Released (g) 63 Student Procedure - Part 2: 1. Using your TBB, find the mass of the closed system once the chemical reaction has completed. Be sure to keep balloon attached. 2. Record the info into the data table below. 3. Carefully remove the balloon and let all the gases escape. 4. Place the deflated balloon back onto the Erlenmeyer flask. 5. Find the mass again using your TBB. 6. Record your info into the data table above. Explain: Look at the chemical equation below: *NaHCO3 + CH3COOH → NaOOCCH3 + H20 + CO2 Baking + Vinegar → Soda 1. 2. 3. 4. Sodium + Water + Carbon Acetate Dioxide Name the reactants:_______________________________________________________ Name the products:_______________________________________________________ Name the gas produced:___________________________________________________ Compare the mass of the closed system before and after the reaction. Explain your results. 5. Were any new elements introduced into the closed system? Where did the gas come from? Explain. 6. What evidence did you observe to indicate that a chemical reaction took place? 7. After the gas was released, what happened to the mass of the system and why? 8. Did your results support this statement? Why/Why Not? EL8_2014 64 Student Conclusion Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 65 Teacher CARBON CYCLE STATION GAME (Adapted from Resources for Educators from the National Center for Atmospheric Research) http://www.ucar.edu Next Generation Sunshine State Standards Benchmark: SC.8.L.18.3 Construct a scientific model of the carbon cycle to show how matter and energy are continuously transferred within and between organisms and their physical environment. SC.8.N.1.5 Analyze the methods used to develop a scientific explanation as seen in different fields of science. Background Information for the teacher: The movement of carbon through various aspects of the natural environment is the focus of much scientific research. Global warming and climate change can be attributed to the increased amount of heattrapping gases, such as carbon dioxide. Students must develop an understanding of how carbon moves through the environment in order to appreciate the complexity of developing solutions to address problems associated with climate change. In addition, since anthropogenic influences impact how much carbon is reintroduced to the active carbon cycle, students should recognize that human actions negatively affect the environment. Materials: 7 Dice 7 Station Signs 7 Station Movement Directions Carbon Cycle Passport for Each Student Carbon Atom Model for Each Student Blank Bar Graph for Each Student Engage: Dirt for Lunch 1. Have students list everything they are having or had for lunch. 2. Ask students if they can name a food in their lunch that did not come from dirt? Mention that no matter what you will eat or have eaten for lunch, ultimately they are eating dirt! 3. Have students create a concept map to attempt to figure out the ingredients in different foods and, as a group, trace each food’s origin back to the Earth. 4. Use a tuna fish sandwich for an example. The bread came from wheat grown in the dirt. Pickles are preserved cucumbers grown in the dirt. Lettuce was grown in the dirt. Mayonnaise came from eggs, which came from chickens that ate grains grown in the dirt. Tuna living in the ocean eat smaller fish that eat zooplankton that eat phytoplankton, which need nutrients from the decomposed bodies of dead plants and animals accumulated on the ocean floor and brought to the surface by currents. 5. Optional: As a group create a poster using an appropriate graphic organizer explaining “I Eat Dirt…Ask Me How”. Drawings, magazine cutouts, or computer graphics should be incorporated into the poster. 6. Optional: Studyjams-Carbon Cycle, TED Ed-Carbon Cycle, BBC-Carbon Cycle Explore: Teacher Preparation: EL8_2014 66 Teacher Print and laminate station signs for durability. Place signs outside next to real life examples. For example place “plant” station by flowers, plants or grass. Place “soil” station on the ground, etc. Place one die at each station. Activity: 1. Tell students that they are going to be carbon atoms moving through the carbon cycle. 2. Using the carbon atom model, have students draw in the protons, neutrons and electrons. 3. Students then wear the carbon atom model as they travel the Carbon Cycle. 4. Categorize the places carbon can be found into these stations: Atmosphere, Plants, Animals, Soil, Ocean, Deep Ocean, and Fossil Fuels. Point out the areas outside or in the room that are labeled with each station and contain the directions for movement from that station. 5. Assign students to each station randomly and evenly. Have students identify the different places carbon could go from that given station. Discuss the processes that allow for the transfer of carbon between stations. Students should make a line and roll the die individually to follow the directions for movement from (or retention at) each station. Remind them that they are representing atoms of carbon moving through the carbon cycle and that they should record their movements on the data sheet. 6. Students will realize the routine movements (or non-movements) in the carbon cycle. 7. Once the carbon atoms (students) have had a chance to roll the die ten times, have each student create a bar graph using the data they collected. The bar graph should represent the number of times the carbon atom (student) was at each station. 8. Using graph paper, create a large bar graph recording the number of carbon atoms (students) at each station. EL8_2014 67 Student Name: __________________________________________Period: _______ Date: ________ TRAVEL THE CARBON CYCLE Start Location: ________________________ Trip 1: Where I’m going How I’m getting there: Trip 6: Where I’m going How I’m getting there: Trip 2: Where I’m going How I’m getting there: Trip 7: Where I’m going How I’m getting there: Trip 3: Where I’m going How I’m getting there: Trip 8: Where I’m going How I’m getting there: Trip 4: Where I’m going How I’m getting there: Trip 9: Where I’m going How I’m getting there: Trip 5: Where I’m going How I’m getting there: Trip 10: Where I’m going How I’m getting there: EL8_2014 68 Title: Teacher EL8_2014 69 Teacher CARBON ATOM MODEL Nucleus EL8_2014 70 Teacher The Carbon Cycle THE ATMOSPHERE You are currently a molecule of carbon dioxide in the atmosphere. If you roll… Then you … 1 Stay in the atmosphere. Much of the carbon dioxide in the atmosphere moves through the atmosphere. 2 Go to plant. You are used by a plant in photosynthesis. 3 Stay in the atmosphere. Much of the carbon dioxide in the atmosphere moves through the atmosphere. 4 Stay in the atmosphere. Much of the carbon dioxide in the atmosphere circulates through the atmosphere. 5 Go to surface ocean. 6 Go to plant. You are used by a plant in photosynthesis. EL8_2014 71 Teacher The Carbon Cycle PLANTS BIOSPHERE You are currently a carbon molecule in the structure of the plant. If you roll… Then you … 1 Go to soil. The tree shed its leaves. 2 Stay in plant. You are a carbon molecule in the tree’s trunk. 3 Go to animal. The leaves and berries that the plant produced contain your carbon molecule and were eaten. 4 Stay in plant. You are a carbon molecule in the tree’s roots. 5 Stay in plant. You are a carbon molecule in the tree’s branches. 6 Stay in plant. You are a carbon molecule in the tree’s trunk. EL8_2014 72 Teacher The Carbon Cycle ANIMALS BIOSPHERE You are currently a molecule of carbon in an animal. If you roll… Then you … 1 Stay in animal. The carbon molecule is stored as fat in the animal. 2 Go to soil. The animal that consumed you died and your carbon molecule is returned to the soil. 3 Go to atmosphere. The animal that consumed you respired (breathed) you out as carbon dioxide. 4 Stay in animal. You are eaten by a predator. 5 Go to atmosphere. The animal that consumed you respired (breathed) you out as carbon dioxide. 6 Go to atmosphere. The animal that consumed you respired (breathed) you out as carbon dioxide. EL8_2014 73 Teacher The Carbon Cycle SOIL GEOSPHERE You are currently a molecule of carbon dioxide in the soil. If you roll… Then you … 1 Stay in the soil. Much of the carbon in the soil is stored there. 2 Go to plant. You are used by a plant in photosynthesis. 3 Go to fossil fuels. Your carbon molecule has been in the soil so long it turns into fossil fuels. 4 Go to the atmosphere. 5 Stay in the soil. 6 Go to fossil fuels. Your carbon molecule has been in the soil so long that it turns into fossil fuels. EL8_2014 74 Teacher The Carbon Cycle SURFACE OCEAN HYDROSPHERE You are currently a molecule of carbon dioxide in the surface ocean. If you roll… Then you … 1 Go to deep ocean. 2 Stay in the surface ocean. 3 Go to deep ocean. Your carbon atom was part of an ocean organism that has died and has sunk to the bottom of the ocean. 4 Stay in the surface ocean. 5 Go to the atmosphere. 6 Go to the atmosphere. EL8_2014 75 Teacher The Carbon Cycle DEEP OCEAN HYDROSPHERE You are currently a molecule of carbon in the deep ocean. If you roll… Then you … 1 Stay in the deep ocean. 2 Stay in the deep ocean. 3 Go to surface ocean. 4 Go to surface ocean. 5 Go to surface ocean. 6 Go to animal. An organism in the water has taken you up as food in the deep ocean. EL8_2014 76 Teacher The Carbon Cycle FOSSIL FUELS GEOSPHERE Fossil fuels are a rich source of energy that has been created from carbon that has been stored for many millions of years. If you roll… Then you … 1 Stay in the fossil fuels. 2 Stay in the fossil fuels. 3 Stay in the fossil fuels. 4 Stay in the fossil fuels. 5 Go to the atmosphere. Humans have pumped the fuel that you are part of out of the ground and have used it to power their cars. 6 Go to the atmosphere. EL8_2014 77 Teacher What is the Carbon Cycle? All living organisms are based on the carbon atom. Unique among the common elements of the Earth's surface, the carbon atom has the ability to form bonds with as many as four other atoms (including other carbon atoms) and to form double bonds to itself. Carbon compounds can be solid, liquid, or gas under conditions commonly found on the Earth's surface. Because of this, carbon can help form solid minerals (such as limestone), 'squishy' organisms (such as plants and animals), and can be dissolved in water or carried around the world through the atmosphere as carbon dioxide gas. The attributes of the remarkable carbon atom make possible the existence of all organic compounds essential to life on Earth. Carbon atoms continually move through living organisms, the oceans, the atmosphere, and the crust of the planet. This movement is known as the carbon cycle. The paths taken by carbon atoms through this cycle are extremely complex, and may take millions of years to come full circle. Modified with permission from Global Climates - Past, Present, and Future, S. Henderson, S. Holman, and L. Mortensen (Eds.). EPA Report No. EPA/600/R-93/126, U.S. Environmental Protection Agency, Office of Research and Development, Washington, DC. pp. 59 - 64. EL8_2014 78 Teacher SCALE OF OUR UNIVERSE MODELING ACTIVITY Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.E.5.3 Distinguish the hierarchical relationships between planets and other astronomical bodies relative to solar system, galaxy, and universe, including distance, size, and composition. (Also assesses SC.8.E.5.1 and SC.8.E.5.2.) SC.7.N.1.5 Describe the methods used in the pursuit of a scientific explanation as seen in different fields of science such as biology, geology, and physics. Purpose of Activity: Students will identify the various celestial bodies in our universe through a hands-on modeling activity. Students will understand relative scales of the various distances of the Universe by incorporating a scale in their model of the universe. Materials (Suggested, but not limited to) Modeling clay String Different sized balls Markers Paper Scissors Balloons Straws Procedures: Before Engage: Activity Teacher will project http://htwins.net/scale2/ to introduce how large our universe is and all that is inside. Teacher will ask students what else they think is inside the universe and how long it would take to reach the outskirts of our solar system. During Explore Activity Teacher must explain expectations and directions for activity: Students will be given a list of celestial objects they are to include in their model (see student handout). Students will work in groups of 2-3 to create a scale to use to illustrate distances between the objects. Students will gather any materials they wish to use to create their model of the universe. Explain Students will explain how they created their universe model. Students will demonstrate their understanding of the scale of the universe by explaining the different celestial bodies and how far apart they are based on the scale they used in their model. After Elaborate Activity After activity, students will complete activity write up and discuss the benefits and limitations of their model. Evaluate Teacher will evaluate understanding of objectives based on student conclusions in C-E-R. EL8_2014 79 Student SCALE OF OUR UNIVERSE MODELING ACTIVITY Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.E.5.3 Distinguish the hierarchical relationships between planets and other astronomical bodies relative to solar system, galaxy, and universe, including distance, size, and composition. (Also assesses SC.8.E.5.1 and SC.8.E.5.2.) SC.7.N.1.5 Describe the methods used in the pursuit of a scientific explanation as seen in different fields of science such as biology, geology, and physics. Celestial Body Earth (planet) Moon Mars (planet) Neptune (planet) Asteroid Belt Sun Betelgeuse Star Andromeda Galaxy What’s in our Universe? Distance from Earth 0 384,400 km 225,000,000 km 4,300,000,000 km 255,000,000 km 149,600,000 km 6,050,000,000,000,000 km 24,000,000,000,000,000,000 km Problem Statement Materials Procedures (Plan of Model) Build, draw, or map out your model on a separate paper. EL8_2014 80 Student Conclusion Create a claim, support with evidence, and explain with reasoning based on ONE of the following questions below: 1) What are the benefits and limits of your model of the universe? 2) How would you describe the vastness (largeness) of our universe? Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 81 Teacher STAR BRIGHT APPARENT MAGNITUDE LAB Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.E.5.5 Describe and classify specific physical properties of stars: apparent magnitude (brightness), temperature (color), size, and luminosity (absolute brightness). AA (Cognitive Complexity: Level 2: Basic Application of Skills & Concepts) SC.8.N.1.1 Define a problem from the 8th grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of various types: systematic observations, or experiments, identify variables. AA (Cognitive Complexity: Level 3: Strategic Thinking & Complex Reasoning) Purpose: Students will demonstrate how distance affects the apparent magnitude and absolute brightness of a flashlight and relate it to brightness of stars. Students will explain the apparent magnitude and absolute brightness of a star. Students will classify stars based on shared characteristics. Materials (per group): 3 pencils 1 meter stick Tape 2 flashlights Procedures: Preparation: Prepare materials Before Activity During Activity EL8_2014 Engage: Teacher shows the Discovery Education Video on Brightness & Luminosity http://app.discoveryeducation.com/player/view/assetGuid/E9FF8166-9C574D00-A9D0-169D2845F09E Teacher reviews vocabulary and clarifies the meaning of “absolute brightness” and “apparent magnitude” as the terms to use. Teacher will ask students what other things emit light and are bright, such as stars to transition to lab activity. Explore: Teacher will pass out lab handouts and students will read background information. Students will answer the pre-lab questions and teacher will begin a discussion on the purpose of the lab. Students will work in groups of 3 to execute the lab activity. Teacher will monitor groups and ask students the following questions: 1. How does distance of the flashlight affect what you see? The closer the flashlight is, the brighter it appears; and the further away the flashlight is the dimmer it appears. 2. When the flashlights are the same distance from you, what do you see and why? When the flashlights are both close and both far their brightness is the same. This is because both flashlights are emitting the same amount of brightness since they are the same flashlight. 3. How does this activity relate to our objective and our knowledge about stars? This activity shows us how distance affects the observed brightness of 82 Teacher a star. For example, stars that are really far away may be bright but don’t seem bright because of their distance. Stars that are closer to Earth seem brighter because there is less distance between the star and the Earth. Explain: Students write their explanations and conclusions in their lab handout Students will answer the question: “What is the relationship between a star’s apparent magnitude and distance from Earth?” in the C-E-R template After Activity Elaborate After students complete the Apparent Magnitude lab and there is sufficient time, the teacher may pass out the following worksheet taken from www.middleschoolscience.com to reinforce concepts related to brightness of stars: http://www.middleschoolscience.com/magnitude.pdf Evaluate Teacher will evaluate student understanding and mastery of concept based on responses for conclusion of lab. EL8_2014 83 Student “STAR BRIGHT” Apparent Magnitude Lab Purpose: To demonstrate how distance affects the apparent magnitude and absolute brightness of an object. Background: Stars vary widely in brightness. Some appear very bright, while others are barely visible to the naked eye. Around 150 B.C., long before the invention of telescopes, the Greek astronomer Hipparchus devised a scale to measure apparent magnitude, the brightness of stars as seen with the naked eye from Earth. He gave a value of 1 to the brightest star and a value of 6 to the dimmest. Today, we use a variation of his scale to measure the brightness of stars. Instead of observing and estimating magnitudes with the naked eye, we now use an instrument called a photometer, which produces more precise measurements. Also, the scale has been extended beyond 1 to 6 so astronomers can measure an even broader range of brightness. In this project, you will demonstrate the effect of luminosity (absolute brightness) and distance on the apparent magnitude of a star. You will build an instrument to measure apparent magnitude. You will learn how apparent magnitude differs from intrinsic (natural) luminosity, which is the amount of light a star emits. You will also discover the difference between apparent and absolute magnitude. Materials 3 pencils Meter stick tape 2 identical incandescent flashlights with new batteries Procedures 1. Assign group members their roles. 2. Tape pencil 1 to the ground to mark the starting point of this lab. 3. Measure 3 meters from pencil 1 and tape pencil 2 to the ground. 4. Measure 3 meters from pencil 2 and tape pencil 3 to the ground. Pencil 3 should be 6 meters from pencil 1. 5. Turn off the lights, stand beside pencil 1. 6. Instruct two of your teammates to hold flashlights and to stand side by side at Pencil 2. 7. Instruct your two teammates to turn on their flashlights and shine them toward you. 8. Look at the lights just long enough to compare their brightness and record your observations. 9. Ask one of your teammates to move to Pencil 3, while continuing to shine the light toward you. 10. Compare the brightness of the lights and record your observations. 11. Ask your other teammate to move to the third pencil while continuing to shine the light toward you. 12. Compare the brightness of the lights and record your observations. Pre-Lab Questions What is the purpose of this lab? What is the difference between absolute brightness and apparent magnitude of a star? EL8_2014 84 Student Observation Notes Flashlights turned on from Pencil 2 Flashlight 1 on Pencil 2 Flashlight 2 on Pencil 3 Flashlights turned on from Pencil 3 Post-Lab Analysis Based on your observations and understanding of apparent magnitude and luminosity, make a claim that answers these questions: What is the relationship between a star’s apparent magnitude and distance from Earth? CLAIM – A statement that describes what you think is happening. EVIDENCE – Facts and observations that support your claim. REASONING – An explanation that ties your evidence to your claim. EL8_2014 85 Teacher THE MARTIAN SUN-TIMES Next Generation Sunshine State Standards Benchmark: SC.8.E.5.7 Compare and contrast the properties of objects in the Solar System including the Sun, planets, and moons to those of Earth, such as gravitational force, distance from the Sun, speed, movement, temperature, and atmospheric conditions. (AA)(Also assesses SC.8.E.5.4 and SC.8.E.5.8.). Background Information for the teacher: Sources: NASA.gov and http://nineplanets.org/mars.html Our Solar system is a part of a spiral galaxy called the Milky Way. It is comprised of our nearest star, the Sun, and the celestial bodies that surround it. There are eight (8) planets in our solar system – Pluto was downgraded to a dwarf planet in 2006 mainly because it orbits around the Sun in “zones of similar objects that can cross its path.” Pluto has a more distinguished recognition because dwarf planets orbiting the Sun beyond Neptune are referred to as plutoids. Of the eight remaining planets, there are four (4) inner “rocky” planets and four (4) outer “gas giants.” One of particular interest, is Mars. Mars (Greek: Ares) is the god of War. The planet probably got this name due to its red color; Mars is sometimes referred to as the Red Planet. (An interesting side note: the Roman god Mars was a god of agriculture before becoming associated with the Greek Ares; those in favor of colonizing and terraforming Mars may prefer this symbolism.) The name of the month March derives from Mars. Mars has been known since prehistoric times. Of course, it has been extensively studied with groundbased observatories. But even very large telescopes find Mars a difficult target, it's just too small. It is still a favorite of science fiction writers as the most favorable place in the Solar System (other than Earth!). Early in its history, Mars was much more like Earth. As with Earth almost all of its carbon dioxide was used up to form carbonate rocks. But lacking the Earth's plate tectonics, Mars is unable to recycle any of this carbon dioxide back into its atmosphere and so cannot sustain a significant greenhouse effect. The surface of Mars is therefore much colder than the Earth would be at that distance from the Sun. Materials: Computer with Internet access, various spherical objects of different sizes (i.e., basketball, softball, soccer ball, large marbles small marbles, beads, etc.) Objectives Students will: Explore the solar system Gather, interpret, and compare current weather information for Mars and Earth. Interpret and make inferences from data. Teacher note: Students are told that they are Earthling weather/news reporters for an Internet newspaper called the Martian Sun-Times. They will write articles for the newspaper comparing weather and/or life on Mars and Earth. It is recommended that you assign a team to each investigation. It is possible for students to collect data and answer the questions in one period if there is a computer for each group. Another period will be necessary for them to discuss and write their article. Encourage students to use their factual information but to consider one of the following formats when writing their articles: travel brochure, human or Martian interest - story, fashion report, disaster report, weather predictions, etc. Students will be evaluated on the basis of effort, job performance, team participation and their literary contribution. Your role will be to answer questions for students and assist students in their interpretations. As always is the case, it's important for you to have done the investigations before teaching them. Occasionally, you may need to further explain some science concept found in the "Stats" sheets. EL8_2014 86 Teacher Part 1: Solar System Sizes 1. As a class, discuss the actual size of our solar system – the planets, moons, and the Sun. Note that all of the measurements in the table below are in thousands, and even hundreds of thousands, of kilometers. 2. Using a spreadsheet program or calculator, begin to calculate the needed data in column 3. Once done, discuss these ratios as a class. 3. To complete column 4, set Earth’s diameter to the size of a large marble and recalculate the sizes based on the ratios in column 3. 4. Try to think of objects that correspond to the calculated sizes. Answers Solar System Body Equatorial Diameter (kilometers) Diameter Compared with Earth's Scaled Diameters Scaled to… Earth=Large Marble (cm) Mercury 4,880 0.38 0.76 Venus 12,100 0.95 1.9 Earth 12,756 1 2 Mars 6,787 0.53 1.06 Jupiter 143,200 11.2 22.4 Saturn 120,000 9.4 18.8 Uranus 51,800 4.1 8.2 Neptune 49,528 3.9 7.8 Pluto (Dwarf planet) ~2,330 0.19 0.38 Moon 3,476 0.27 0.54 Sun 1,392,000 109 218 Everyday Object Representing Solar System Body Small bead Large marble Large Marble Small marble Basketball Soccer ball Softball Softball Tiny bead Tiny bead Giant beach ball? (Very Large) Source(s) www.perkins-observatory.org and www.flpromise.org Part 2 - Solar System Distance Scale Model Objective: Students will use mathematical equations, measuring tools and skills to create an accurate scale model of the solar system. Background Information: Distances in space can sometimes be hard to imagine because space is so vast. Think about measuring the following objects: a textbook, the classroom door, or the distance from your house to school. You would probably have to use different units of measurement. In order to measure long distances on Earth, we would use kilometers. But larger units are required for measuring distances in space. One astronomical unit equals 150 million km (1 AU = 150,000,000 km), which is the average distance from the Earth to the Sun. EL8_2014 87 Teacher Materials: - receipt paper rolls (adding machine tape) or old VHS tape - meter stick - metric ruler - markers or colored pencils - scissors Engage: Ask students to brainstorm about all of the objects that they have seen or observed in the night sky. Then discuss with the class how far away they think these objects (stars, planets, or satellites) are. Reinforce to students that there are planets much closer to the Earth than stars other than our Sun. Explore 1. As a class, decide what scale you will use to determine your measured distance from the Earth to the Sun. This measurement will represent one Astronomical Unit (AU); (Ex: 10 cm = 1 AU). 2. Multiply your chosen AU standard by 40 to determine the length of adding machine tape needed to complete your scale model activity. (10 cm x 40 = 400 cm of tape). 3. Place your values in Table 2. 4. Cut the adding machine tape to the appropriate length. Note: If you would like to include the Sun and Asteroid Belt, be sure to cut extra length (5 cm – 7cm should be adequate) at the start of your distance scale model. Students should also consider that the Sun’s size will not be to scale. 5. Mark one end of the tape to represent the Sun. 6. Measure from the edge of your group’s drawn Sun the distances for each planet. Place a dot where each planet should be placed. Include your scale on the model. 7. Once all of the planets have mapped out, each group member should choose one or two planets to draw and color. Use your textbook or materials provided by your teacher as a reference. EL8_2014 88 Teacher PLANET TABLE 2: Scaled Distances of Planets Distance from the Standard-Scale Sun in (chosen by Astronomical class/group) Units (AU) AU x scale unit Mercury 0.4 Venus 0.7 Earth 1.0 Mars 1.5 Jupiter 5.2 Saturn 9.5 Uranus 19.5 Neptune 30.2 Pluto (Dwarf Planet) 40 Distance of Planet in the chosen scale. (cm) Results and Conclusions: 1. Why do you think scale models are important? 2. Why were you instructed to multiply the distances in AU by 40 to determine how long your scale model needed to be? 3. Compare and contrast the distances of the inner and outer planets from the Sun Extension: 4. Draw the planets by scale according to size (diameter) on the distance scale model. 5. Research other celestial bodies in the universe (other known stars and galaxies). Using AU and units such as a light year, include these in you distance scale model. Part 3 - Martian Sun Times Reporters Teacher’s Procedure: 1. Divide the class into seven different groups. Each person within the group will be assigned a specific job, e.g. secretary, researcher(s), editor, organizer. 2. Assign to each group one of the investigations to research. Use the factual information obtained to prepare an article. This may consist of anyone of a variety of formats, e.g., a newspaper article, a travel brochure, a human –interest (or Martian interest) story, a fashion report, weather predictions. EL8_2014 89 Teacher Student Procedure: 1. Your group will be assigned an investigation to research and present to class. 2. Use the factual information obtained to prepare an article. This may consist of anyone of a variety of formats, e.g., a newspaper article, a travel brochure, a human –interest (or Martian interest) story, a fashion report, weather predictions. 3. Each person within the group will be assigned a specific job, e.g. secretary, researcher(s), editor, organizer. Summary of Investigations: Investigation I: Weather Forecasts for Earthlings and Martians. (Comparing weather for Mars and where you live). Compare temperatures and wind speeds on Mars and on Earth where you live, as well as noting the temperature ranges across the two planets. Investigation II : A Martian Summer Day (Comparing temperatures for summer on Mars and the place you live) Research the typical high and low summer temperatures for Mars. Compare temperatures for the current date on Mars and Earth based upon 30° N latitude. Investigation III: Stormy Mars: Dust Gets In My Eyes (Finding out about dust storms on Mars). Discover the effect of Martian dust storms on temperatures. Find out what might cause the storms and infer the length of one storm. Investigation IV: Probing Earth and Mars: What Should We Pack? (Finding out temperatures at various landing sites) If MASA (Martian Aeronautics and Space Administration) sent astronauts to Earth to places that match the latitude and longitude of Viking and Pathfinder landing sites, where would they land and what weather conditions would they encounter? Investigation V: Life on Mars: Where's the Party? (Finding out about the possibility of life on Mars) Learn about the Martian meteorite that may show evidence of life there. Are any temperatures on Mars similar to Earth? Considering the environment of Mars what, would a Martian look like? Investigation VI: Getting to Mars: Are We There Yet? (Finding out about Mars' orbit and NASA Missions) Learn about planetary orbits and interplanetary travel. How long would a trip from Earth to Mars take? What are some of the next Martian missions planned? Investigation VII: Exploring Mars: Oh Water, Where Art Thou? (Finding out about water on Mars) Early observers of Mars thought they saw canals on the planet. There are no canals, but there is a lot of evidence of once– abundant water on Mars. Students will see current Mars images and compare them to water– formed features on Earth. Extension: 1. Allow students to imagine that they are living on one of the planets other than Earth. They must assume the role of a travel agent who is trying to attract visitors to their home world. They must create an Interplanetary Travel Brochure. Resources: http://www.ucls.uchicago.edu/MartianSunTimes/index.html) http://www.nineplanets.org/mars.html EL8_2014 90 Student THE MARTIAN SUN-TIMES Next Generation Sunshine State Standards Benchmark: SC.8.E.5.7 Compare and contrast the properties of objects in the Solar System including the Sun, planets, and moons to those of Earth, such as gravitational force, distance from the Sun, speed, movement, temperature, and atmospheric conditions. (AA)(Also assesses SC.8.E.5.4 and SC.8.E.5.8.). Objectives Students will: Explore the solar system Gather, interpret, and compare current weather information for Mars and Earth. Interpret and make inferences from data. Materials: Computer with Internet access, various spherical objects of different sizes (i.e., basketball, softball, soccer ball, large marbles small marbles, beads, etc.) Part 1: Solar System Sizes 1. As a class, discuss the actual size of our solar system – the planets, moons, and the Sun. Note that all of the measurements in the table below are in thousands, and even hundreds of thousands, of kilometers. 2. Working in groups of 3 students, use a spreadsheet program or calculator, begin to calculate the needed data in column 3. Divide each equatorial diameter by Earth’s diameter. Once done, discuss these ratios as a class. 3. To complete column 4, set Earth’s diameter to the size of a large marble and recalculate the sizes based on the ratios in column 3. (multiply Diameter compared with Earth x Earth’s Scaled Diameter) 4. Try to think of objects that correspond to the calculated sizes. 5. Arrange the planets in order, be sure to identify asteroid belt, inner planets, and outer planets. 6. Complete the discussion questions. Table 1: Ratio of the diameters of the other bodies compared with Earth's diameter. Diameter Scaled Diameters Everyday Object Equatorial Scaled to… Compared Representing Solar System Body Diameter Earth=Large Marble with Solar System (kilometers) (cm) Earth's Body Mercury 4,880 Venus 12,100 Earth 12,756 Mars 6,787 Jupiter 143,200 Saturn 120,000 Uranus 51,800 Neptune 49,528 Pluto (Dwarf planet) ~2,330 Moon 3,476 Sun 1,392,000 EL8_2014 1 2. Large Marble 91 Student Discussion Questions 1. Identify the following: a. Inner planets b. Outer planets c. Dwarf planet d. Moon e. Star 2. Compare and contrast the sizes of the planets, moon, and stars EL8_2014 92 Student Part 2 - Solar System Distance Scale Model Objective: Students will use mathematical equations, measuring tools and skills to create an accurate scale model of the solar system. Background Information: Distances in space can sometimes be hard to imagine because space is so vast. Think about measuring the following objects: a textbook, the classroom door, or the distance from your house to school. You would probably have to use different units of measurement. In order to measure long distances on Earth, we would use kilometers. But larger units are required for measuring distances in space. One astronomical unit equals 150 million km (1 AU = 150,000,000 km), which is the average distance from the Earth to the Sun. Materials: - receipt paper rolls (adding machine tape) or old VHS tape - meter stick - metric ruler - markers or colored pencils - scissors Explore 1. As a class, decide what scale you will use to determine your measured distance from the Earth to the Sun. This measurement will represent one Astronomical Unit (AU); (Ex: 10 cm = 1 AU). 2. Multiply your chosen AU standard by 40 to determine the length of adding machine tape needed to complete your scale model activity. (10 cm x 40 = 400 cm of tape). 3. Place your values in Table 2. 4. Cut the adding machine tape to the appropriate length. Note: If you would like to include the Sun and Asteroid Belt, be sure to cut extra length (5 cm – 7cm should be adequate) at the start of your distance scale model. Students should also consider that the Sun’s size will not be to scale. 5. Mark one end of the tape to represent the Sun. 6. Measure from the edge of your group’s drawn Sun the distances for each planet. Place a dot where each planet should be placed. Include your scale on the model. 7. Once all of the planets have mapped out, each group member should choose one or two planets to draw and color. Use your textbook or materials provided by your teacher as a reference. EL8_2014 93 Student PLANET TABLE 2: Scaled Distances of Planets Distance from the Standard-Scale Sun in (chosen by Astronomical class/group) Units (AU) AU x scale unit Mercury 0.4 Venus 0.7 Earth 1.0 Mars 1.5 Jupiter 5.2 Saturn 9.5 Uranus 19.5 Neptune 30.2 Pluto (Dwarf Planet) 40 Distance of Planet in the chosen scale. (cm) Results and Conclusions: 1. Why do you think scale models are important? 2. Why were you instructed to multiply the distances in AU by 40 to determine how long your scale model needed to be? 3. Compare and contrast the distances of the inner and outer planets from the Sun Extension: 1. Draw the planets by scale according to size (diameter) on the distance scale model. 2. Research other celestial bodies in the universe (other known stars and galaxies). Using AU and units such as a light year, include these in you distance scale model. Part 3 - Martian Sun Times Reporters Student Procedure: 1. Your group will be assigned an investigation to research and present to class. 2. Use the factual information obtained to prepare an article. This may consist of anyone of a variety of formats, e.g., a newspaper article, a travel brochure, a human –interest (or Martian interest) story, a fashion report, weather predictions. 3. Each person within the group will be assigned a specific job, e.g. secretary, researcher(s), editor, organizer. EL8_2014 94 Student Summary of Investigations: Investigation I: Weather Forecasts for Earthlings and Martians. (Comparing weather for Mars and where you live). Compare temperatures and wind speeds on Mars and on Earth where you live, as well as noting the temperature ranges across the two planets. Investigation II : A Martian Summer Day (Comparing temperatures for summer on Mars and the place you live) Research the typical high and low summer temperatures for Mars. Compare temperatures for the current date on Mars and Earth based upon 30° N latitude. Investigation III: Stormy Mars: Dust Gets In My Eyes (Finding out about dust storms on Mars). Discover the effect of Martian dust storms on temperatures. Find out what might cause the storms and infer the length of one storm. Investigation IV: Probing Earth and Mars: What Should We Pack? (Finding out temperatures at various landing sites) If MASA (Martian Aeronautics and Space Administration) sent astronauts to Earth to places that match the latitude and longitude of Viking and Pathfinder landing sites, where would they land and what weather conditions would they encounter? Investigation V: Life on Mars: Where's the Party? (Finding out about the possibility of life on Mars) Learn about the Martian meteorite that may show evidence of life there. Are any temperatures on Mars similar to Earth? Considering the environment of Mars what, would a Martian look like? Investigation VI: Getting to Mars: Are We There Yet? (Finding out about Mars' orbit and NASA Missions) Learn about planetary orbits and interplanetary travel. How long would a trip from Earth to Mars take? What are some of the next Martian missions planned? Investigation VII: Exploring Mars: Oh Water, Where Art Thou? (Finding out about water on Mars) Early observers of Mars thought they saw canals on the planet. There are no canals, but there is a lot of evidence of once– abundant water on Mars. Students will see current Mars images and compare them to water– formed features on Earth. Extension: 2. Imagine that you are living on one of the planets other than Earth. Assume the role of a travel agent who is trying to attract visitors to their home world. Create an Interplanetary Travel Brochure . EL8_2014 95 Teacher WHAT CAUSES THE SEASONS? Benchmark: SC.8.E.5.9 Explain the impact of objects in space on each other including: 1. the Sun on the Earth including seasons and gravitational attraction 2. The Moon on the Earth, including phases, tides, and eclipses, and the relative position of each body. (AA) Fair Game Benchmarks: SC.7.N.1.4 Identify test variables (independent variables) and outcome variables (dependent variables) in an experiment. (Assessed as SC.8.N.1.1) SC.7.N.3.2 Identify the benefits and limitations of the use of scientific models. (Assessed as SC.7.N.1.5 Overview: Because the axis of the Earth is tilted, the Earth receives different amounts of solar radiation at different times of the year. The tilt of the axis of the Earth, as well as the revolution around the sun produces the seasons. In this experiment, a simulated Sun—a light bulb—will shine on a thermometer attached to a globe. You will study how the tilt of the globe influences warming caused by the lighted bulb. Objective: Compare simulated warming of your city by the Sun in the winter and in the summer. Explain the causes of the cycle of seasons on Earth. Materials: Globe of the Earth Tape Metric ruler thermometer Lamp with 100-watt bulb Ring stand and utility clamp 20-cm Length of string Engage: Provide students with visuals of extreme climates and ask if anyone has lived in a climate very different than that of Miami. Discuss possible reasons for the change in seasons. Accept all possible answers from students and readdress ideas at the end of the lab. Common misconception note: Students often believe that the seasons are caused due to the distance of Earth from the Sun and may be aware of the elliptical nature of Earth’s orbit. You may note at the end of the lab, when addressing this misconception that the Northern and Southern hemispheres have opposite seasons, which would disprove the distance from sun hypothesis. Additionally, you may note that Earth is actually farther from the sun during the summer than in the Northern hemisphere. EL8_2014 96 Teacher Explore: Procedure: Figure 1 1. Prepare the light bulb (simulated Sun). a. Fasten the lamp to a ring stand as shown in Figure 1. b. Stand the ring stand and lamp in the center of your work area. c. Position the globe with the North Pole tilted away from the lamp as shown in Figure d. Position the bulb at the same height as the Tropic of Capricorn. Note: The Sun is directly over the Tropic of Capricorn on December 21, the first day of winter. 2. Attach the thermometer to the globe. a. Find your city or location on the globe. b. Tape the thermometer to the globe with the tip of the thermometer at your location. Place the tape about 1 cm from the tip of the thermometer. c. To keep the tip of the thermometer in contact with the surface of the globe, fold a piece of paper and wedge it under the thermometer as shown in Figure 2. 3. Position the globe for winter (in the Northern Hemisphere) data collection. a. Turn the globe to position the North Pole (still Figure 2 tilting away from the lamp), your location, and the bulb in a straight line. b. Cut a piece of string 10-cm long. c. Use the string to position your location on the globe at 10 cm from the bulb (you may position farther, up to 20 cm, depending on the intensity of the lamp that you are using). d. Do not turn on the lamp until after you have recorded the initial temperature. 4. Collect winter data. a. Record the initial temperature. b. After 5 minutes record the final temperature. c. Turn off the light. 5. Record the beginning and final temperatures (to the nearest 0.1°C). 6. Position the globe for summer data collection. a. Move the globe to the opposite side of the lamp. b. Position the globe with the North Pole tilted toward the lamp. Note: This represents the position of the Northern Hemisphere on June 21, the first day of summer. c. Turn the globe to position the North Pole, your location, and the bulb in a straight line. d. Use the string to position your location on the globe 10 cm from the bulb. e. Do not turn on the lamp until after you have recorded the initial temperature. 7. Collecting summer data. a. Let the globe and thermometer cool to the beginning temperature that you recorded for the winter setup. b. When the globe and probe have cooled, begin data collection. c. Record the final temperature after 5 minutes. Turn the lamp off. EL8_2014 97 Teacher Explain: Processing Data: 1. In the space provided in the data table, subtract to find the temperature change for each season. 2. How does the beginning and final temperature change for summer compare to the temperature change for winter? 3. During which season is the sunlight more direct? Explain. 4. What would happen to the temperature changes if the Earth was tilted more than 23.5 degrees? 5. As you move the globe from its winter position to its summer position, the part of the globe closest to the bulb changes. Describe how it changes. 6. What other factors affect the climate in a region? 7. Identify the test variable, outcome variable, and any controlled variables in the experiment. 8. Why is this model useful for understanding the seasons and how is it limited? 9. What improvements can be made to this model of the seasons? Elaboration: Repeat the experiment for locations in the Southern Hemisphere and other areas (different latitudes) in the Northern Hemisphere to develop an understanding of climate zones. Students illustrate the position of the Earth around the sun in an elliptical shape. Students must include the following vocabulary: tilt, rotation, revolution, year, winter, spring, summer, fall, equator, northern hemisphere, southern hemisphere. Possible diagram: EL8_2014 98 Student WHAT CAUSES THE SEASONS? Benchmark: SC.8.E.5.9 Explain the impact of objects in space on each other including: 1. the Sun on the Earth including seasons and gravitational attraction 2. The Moon on the Earth, including phases, tides, and eclipses, and the relative position of each body. (AA) Fair Game Benchmarks: SC.7.N.1.4 Identify test variables (independent variables) and outcome variables (dependent variables) in an experiment. (Assessed as SC.8.N.1.1) SC.7.N.3.2 Identify the benefits and limitations of the use of scientific models. (Assessed as SC.7.N.1.5 Overview: Because the axis of the Earth is tilted, the Earth receives different amounts of solar radiation at different times of the year. The tilt of the axis of the Earth, as well as the revolution around the sun produces the seasons. In this experiment, a simulated Sun—a light bulb—will shine on a thermometer attached to a globe. You will study how the tilt of the globe influences warming caused by the lighted bulb. Objective: Compare simulated warming of your city by the Sun in the winter and in the summer. Explain the causes of the cycle of seasons on Earth. Materials: Globe of the Earth Tape Metric ruler thermometer Lamp with 100-watt bulb Ring stand and utility clamp 20-cm Length of string Problem Statement: How does the tilt of Earth affect the temperature on the Northern and Southern hemispheres? Test Variable (IV): ________________________________________________________________ Outcome Variable (DV): ______________________________________________________ Constants:________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Hypothesis: _____________________________________________________________________________________ _____________________________________________________________________________________ ____________________________________________________________________________________. EL8_2014 99 Student Procedure: Figure 1 1. Prepare the light bulb (simulated Sun). a. Fasten the lamp to a ring stand as shown in Figure 1. b. Stand the ring stand and lamp in the center of your work area. c. Position the globe with the North Pole tilted away from the lamp as shown in Figure. d. Position the bulb at the same height as the Tropic of Capricorn. Note: The Sun is directly over the Tropic of Capricorn on December 21, the first day of winter. 2. Attach the thermometer to the globe. a. Find your city or location on the globe. b. Tape the thermometer to the globe with the tip of the thermometer at your location. Place the tape about 1 cm from the tip of the thermometer. c. To keep the tip of the thermometer in contact with the surface of the globe, fold a piece of paper and wedge it under the thermometer as shown in Figure 2. 3. Position the globe for winter (in the Northern Hemisphere) data collection. Figure 2 a. Turn the globe to position the North Pole (still tilting away from the lamp), your location, and the bulb in a straight line. b. Cut a piece of string 10-cm long. c. Use the string to position your location on the globe at 10 cm from the bulb (you may position farther, up to 20 cm, depending on the intensity of the lamp that you are using). d. Do not turn on the lamp until after you have recorded the initial temperature. 4. Collect winter data. a. Record the initial temperature. b. After 5 minutes record the final temperature. c. Turn off the light. 5. Record the beginning and final temperatures (to the nearest 0.1°C). 6. Position the globe for summer data collection. a. Move the globe to the opposite side of the lamp. b. Position the globe with the North Pole tilted toward the lamp. Note: This represents the position of the Northern Hemisphere on June 21, the first day of summer. c. Turn the globe to position the North Pole, your location, and the bulb in a straight line. d. Use the string to position your location on the globe 10 cm from the bulb. e. Do not turn on the lamp until after you have recorded the initial temperature. 7. Collecting summer data. a. Let the globe and thermometer cool to the beginning temperature that you recorded for the winter setup. b. When the globe and thermometer have cooled, begin data collection. c. Record the final temperature after 5 minutes. Turn the lamp off. EL8_2014 100 Student Data: Test Variable: Hemisphere Northern (Winter) Initial Temperature (C) Final Temperature (C) Temperature Change (C) Southern (Summer) Results:__________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Conclusion: Answer the question this investigation sought to answer in the C-E-R template below. Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 101 EL8_2014 102 Teacher DENSITY OF BLOCKS ACTIVITY Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.N.1.1 Define a problem from the eighth grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of various types, such as systematic observations or experiments, identify variables, collect and organize data, interpret data in charts, tables, and graphics, analyze information, make predictions, and defend conclusions. (AA) SC.8.P.8.3 – Explore and describe the densities of various materials through measurement of their masses and volumes. Assessed as SC.8.P.8.4 – Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. Background Information for the teacher: Density is a basic physical property of any sample of matter. It is much more important than other physical properties such as size or shape, in that the numerical value of density for a pure substance at a particular temperature and pressure is a constant and never changes! The density may be determined in the laboratory if the mass and volume of a sample can be determined. Density may be calculated by dividing the mass by the volume (d = m / V). It also may be thought of as the ratio of the mass to the volume. The density of water is important to know. It is 1.0 g/mL at 40C. In this experiment, the student will measure the mass, volume, and the length of several rocks. They will then use their data to explore the relationship between the mass and volume of the rocks and calculate their density. Material Densities of Common Substances Source: Teacher Developed – Classroom Tested Provide students with the following information: You have been given blocks of equal volume. You may want to provide the Density Block samples or have students make cubes 2.54 cm x 2.54 cm x 2.54 cm 1. Based on the densities of the various substances listed in the data table above, ask students to make predictions whether the block made of the various materials would sink or float in water. Block Prediction (sink or float) Observation (sink or float) Acrylic Aluminum Brass Copper EL8_2014 103 Oak Pine Polypropylene PVC Steel Acrylic Evaluate If two blocks of pine were stacked on top of each other, would they sink or float? Explain. Extensions: 1. Students will explore the density of different liquids and/or solutions, e.g. 5%, 10%, 15% saltwater solution. Discover the relationship between density and the solute concentration. EL8_2014 104 Possible Answers Explain Analysis Questions: 1. Which variable is considered the test variable (independent) variable in this lab activity? Type of rock 2. Which variable (s) is considered the outcome variable (dependent) variable in this lab activity? density 3. If the mass of the rock increases, what could happen to the density of each sample? Increase if volume stays same 4. If the volume of the rock increases, what would happen to the density of each sample? It would stay the same because the mass would also increase 5. Analyze your data: What do you observe about the relationship between mass and volume for the rocks with the larger densities and smaller densities? Give examples from the lab in your explanation. Larger densities have larger mass compared to the object’s volume; smaller densities have larger volume compared to the mass. Examples will vary 6. In terms of density, differentiate between an object which floats in water and an object which sinks in water. An object that floats in water is less dense than the water or less than 1 g/cm3 . An object that sinks has a greater density than water. 7. Show how one would set up a ratio to determine the mass of a substance with a density of 8.4g/mL and a volume of 2.0 mL. Determine the mass. 8.4g/mL = ?g/2.0 mL mass = 16.8 g 8. Show how one would set up a ratio to determine the volume of a substance with a density of 4.0 g/mL and a mass of 8.0 g. Determine the volume. 4.0 g/mL = 8.0 g/?mL volume = 2 mL 9. Based on the results of this lab, explain how unknown substances can be identified or distinguished from one another by using their densities. All substances have a specific density. If the mass and volume can be determined, then the substance can be found by comparing with substances of known densities. Bonus question: 10. Density of water is 1 g/ml or 1.0 g/cm3). What is the volume of a sample of water if the mass is 6g? Explain why this is so easy to figure out (think ratio). The density of water is a 1:1 ratio 6 g would mean 6 mL Evaluate If two blocks of pine were stacked on top of each other, would they sink or float? Explain: The blocks would float. The wood is still less dense than water. For example, if the mass doubles, so does the volume, keeping the density the same. Note: Use real examples for students to measure and test. www.sciencenet.org.uk/.../Chemistry/ StructBond/c00195b.html EL8_2014 105 Student DENSITY OF BLOCKS ACTIVITY Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.N.1.1 Define a problem from the eighth grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of various types, such as systematic observations or experiments, identify variables, collect and organize data, interpret data in charts, tables, and graphics, analyze information, make predictions, and defend conclusions. (AA) SC.8.P.8.3 – Explore and describe the densities of various materials through measurement of their masses and volumes. Assessed as SC.8.P.8.4 – Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. Purpose In this activity, you will measure the mass, volume, and the length of several blocks. Then, use your data to explore the relationship between the mass and volume of the rocks and calculate their density. Material Densities of Common Substances Source: Teacher Developed – Classroom Tested Substance Acrylic Aluminum Brass Copper Oak Pine Polypropylene PVC Steel Water Density (g/cm3) 1.1 – 1.2 2.7 8.4 – 8.8 8.96 0.60 – 0.90 0.35 – 0.50 0.91 – 0.94 1.39 – 1.42 7.9 1.0 Based on the densities of the various substances listed in the data table above, make predictions whether the block made of the various materials would sink or float in water. Block Prediction (sink or float) Observation (sink or float) Acrylic Aluminum Brass EL8_2014 106 Copper Oak Pine Polypropylene PVC Steel Acrylic Analysis Questions: 1. Which variable is considered the test variable (independent) variable in this lab activity? 2. Which variable (s) is considered the outcome variable (dependent) variable in this lab activity? 3. If the mass of the rock increases, what could happen to the density of each sample? 4. If the volume of the rock increases, what would happen to the density of each sample? 5. Analyze your data: What do you observe about the relationship between mass and volume for the rocks with the larger densities and smaller densities? Give examples from the lab in your explanation. 6. In terms of density, differentiate between an object which floats in water and an object which sinks in water. 7. Show how one would set up a ratio to determine the mass of a substance with a density of 8.4g/mL and a volume of 2.0 mL. Determine the mass. 8. Show how one would set up a ratio to determine the volume of a substance with a density of 4.0 g/mL and a mass of 8.0 g. Determine the volume. 9. Based on the results of this lab, explain how unknown substances can be identified or distinguished from one another by using their densities. Bonus question: 10. Density of water is 1 g/ml or 1.0 g/cm3). What is the volume of a sample of water if the mass is 6g? Explain why this is so easy to figure out (think ratio). Evaluate If two blocks of pine were stacked on top of each other, would they sink or float? Explain: EL8_2014 107 DIFFERENTIATED INSTRUCTION: OPEN INQUIRY DENSITY OF MATTER Objectives/Purpose: Determine the physical properties of matter including the density of irregular solids. Demonstrate that regardless of the size of a sample, the density of a substance will always remain the same. Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured Demonstrate Achievement of the following Goals: Develop a problem statement based on a physical property of matter and the size of the sample that you would like to investigate. State your hypothesis. Design an experiment to test your hypothesis. Carry out the experiment you designed. Submit a completed lab report to your teacher. Use the “Claim, Evidence & Reasoning” rubric to defend your claims when writing your conclusion. EL8_2014 108 DENSITY OF ROCKS (Differentiated Lab) Revised by: University of Miami – Science Made Sensible Fellows Florida Sunshine State Next Generation Standards Benchmark: SC.8.P.8.4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. (AA) SC.8.P.8.3 Explore and describe the densities of various materials through measurement of their masses and volumes. Background Information for the teacher: Density is a basic physical property of any sample of matter. It is much more important than other physical properties such as size or shape, in that the numerical value of density for a pure substance at a particular temperature and pressure is a constant and never changes! The density may be determined in the laboratory if the mass and volume of a sample can be determined. Density may be calculated by dividing the mass by the volume (d = m / V). It also may be thought of as the ratio of the mass to the volume. The density of water is important to know. It is 1.0 g/mL at 4oC. In this experiment, the student will measure the mass, volume, and the length of several rocks. They will then use their data to explore the relationship between the mass and volume of the rocks and calculate the rocks’ density. Time Frame: 1-1.5 hours Materials: Demonstrations Vegetable oil Karo syrup 1 can of coke 1 can of diet coke Aquarium/container to float cokes Lab Activity Rocks, four types, including pumice stone Plastic baggies or other container for rocks triple beam scales 500ml graduated cylinders Dry ice Container for dry ice demo Bubble wand and soap 1 large graduated cylinder (~1000ml) 250ml Flasks Eye droppers Paper towels Food Coloring dye (for demo also) Pre-lab preparation: 1) Color the water/oil/karo syrup demo with food coloring 2) Select 4 rocks with very different densities as available. One should be pumice stone. Alter the comic strip and student worksheet (clues) so that the “evidence” rock density matches the density of one of the types of rock you have available. EL8_2014 109 3) Gather and prepare demonstration supplies as desired. Engage: 1) Engage the students by discussing the topic of density as a class, explaining how it is a relationship between mass and volume. 2) Perform one or more of the following demonstrations: a. Water/Oil/Syrup layering: Discuss with the class what you will be doing, and have them make predictions of how the three liquids will layer in the 1000ml graduated cylinder. Start with ~250ml of colored water in the cylinder, then add vegetable oil (~100ml) and finally add Karo syrup (~100ml). Discuss why the fluids became layered. b. Coke vs. Diet Coke: Explain what you are going to do, and have your class predict whether the sodas will sink or float. In a clear container (aquarium) filled with water, place a regular coke or comparable soda. Discuss why the soda sank. Next, add the diet coke (it will float). Discuss why a can with the exact same volume will float because it has less mass and therefore is less dense. c. Dry ice/bubbles: In a container that is at least 12 inches deep, place the dry ice. Add some water to speed up the sublimation process and make the gas visible to the students. Then, blow bubbles gently on top of the CO2 gas. Discuss with your class why the bubbles did not sink through the CO2, and how density applies to gases also. (this is also useful at the end of the lab as they elaborate on the concept of density) 3) Engage the students further by reading the “CSI: Following the Hard Evidence” comic (source: http://www.pixton.com/SciMadeSensible). Explore: 1) Give the student all the supplies and the procedures worksheet. Discuss the concept of volume displacement for determining the volume of non-geometric items. 2) Have student complete the procedures while you assist and answer questions. You may need to help them measure the volume of the pumice stone by pushing it completely under the surface of the water using a pencil. Explain: 1) Have students complete the analysis questions at the end of the lab. 2) Discuss any questions as a class. Elaborate/Extension: 1) Students can explore the density of objects with identical masses, but different volumes. Discover the relationship among mass, volume, and density. 2) Students can explore the density of different liquids and/or solutions, e.g. 5%, 10%, 15% saltwater solution. Discover the relationship between density and the solute concentration. This is a good time to do the dry ice demo in order to elaborate that the property density applies to gases also. EL8_2014 110 EL8_2014 111 EL8_2014 112 C.S.I. Density of Rocks: Following the HARD EVIDENCE Goal: Determine the densities of 4 different types of rocks in order to match the “hard evidence” found at the crime scene. Overview: The density of each rock will be calculated by using volume displacement and measuring mass Procedures 1) Look at the rocks and make a prediction about which one you think is the most dense or the least dense. Record your hypothesis, independent variable, and dependent variable, controlled variables and control. 2) Remove your rocks from the evidence bag. 3) Measure the mass of each rock on the balance, record it on this worksheet. 4) Pour 150ml of water from the 500ml beaker into the graduated cylinder. Use the dropper to adjust it exactly to 150ml. This is the INITIAL VOLUME. 5) Place one rock into the graduated cylinder, then determine the volume of water in the cylinder by looking at the BOTTOM OF THE MENISCUS. Record this FINAL VOLUME on your worksheet. 6) Remove the rock by pouring the water back into the beaker and catching the rock with one hand so it doesn’t break the glass. Try not to spill!! 7) Refill the graduated cylinder to 150ml, add the next rock, measure the volume, and record it on your worksheet. Repeat for the third and fourth rocks, drying them with a paper towel and putting them back into their bags. 8) Calculate the volume of each rock by subtracting the initial volume of water (150ml) from the final volume of the water with the rock. Record this on your worksheet. 9) Calculate the density of each rock on the worksheet (Density= Mass/Volume). 10) Answer the questions under the data table on your worksheet and write a conclusion. CLUES: 1) The detectives found a rock at the crime scene that had a density of _____ grams/cm3 2) There are three suspects, each live in an area with a different type of rock. 3) The equation for density is: density= mass/volume Data Table Rock Mass (g) Final Volume (water +rock) Rock Volume (final volumeinitial volume) Density (D=m/v) Creepy Carl Suspicious Susan Naughty Nathan Police Station EL8_2014 113 Questions: 1) Which rock most closely matched the density of the evidence found at the crime scene? 2) Did all the rocks sink? If not, what can you tell about the density of that rock without doing any calculations? 3) For the rock that didn’t sink, if you put a larger sample in the water, would it sink? Why or why not? 4) If you start with 100ml of water, how many grams of Naughty Nathan’s rock would you need to add to your graduated cylinder to increase the volume by 100ml? (remember the equation for density is density=mass/volume, use the density you calculated above) 5) If the mass of the rock increases, what could happen to the volume of each sample? 6) If the volume of the rock increases, what could happen to the mass of each sample? 7) Explain density in terms of a ratio. Give examples from the lab in your explanation. 8) What is the volume of a sample of water if the mass is 6.7g? Explain why this is so easy to figure out. 9) Show how one would set up a ratio to determine the mass of a substance with a density of 5.6g/mL and a volume of 3.7 mL. Then determine the mass. 10) Show how one would set up a ratio to determine the volume of a substance with a density of 2.6 g/mL and a mass of 5.5 g. Then determine the volume. Conclusion: Write a conclusion using the “Claim, Evidence and Reasoning” format. EL8_2014 114 EL8_2014 115 MASS, VOLUME, DENSITY (Comprehensive Science 3 Advanced) Florida Next Generation Sunshine State Standards Benchmark: SC.8.N.1.1 Define a problem from the eighth grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of various types, such as systematic observations or experiments, identify variables, collect and organize data, interpret data in charts, tables, and graphics, analyze information, make predictions, and defend conclusions. (AA) SC.8.P.8.3 – Explore and describe the densities of various materials through measurement of their masses and volumes. Assessed as SC.8.P.8.4 – Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. (AA) Background Information: Density is a basic physical property of any sample of matter. It is much more important than other physical properties such as size or shape, in that the numerical value of density for a pure substance at a particular temperature and pressure is a constant and never changes! The density may be determined in the laboratory if the mass and volume of a sample can be determined. Density may be calculated by dividing the mass by the volume (d = m / V). It also may be thought of as the ratio of the mass to the volume. The density of water is important to know. It is 1.0 g/mL at 4 ºC. In this experiment, the students will measure the mass and volume of several materials. They will then use their data to explore the relationship between the mass and volume of the materials and calculate their density. Literature Connection: “Archimedes and the King’s Crown” Time Frame: 1 hour Materials (per pair of students): Safety goggles 50 mL of isopropyl alcohol (colored red) 50 mL of water (colored blue) 50 mL of salt-water (colored green) Graduated cylinder Eye dropper Calculator Electronic balance or triple-beam balance Procedure Part A: Teacher Pre-Lab Preparation and Presentation 1. Color the isopropyl alcohol red by adding a few drops of red food coloring. 2. Color the water blue by adding a few drops of blue food coloring. 3. Prepare a saltwater solution by mixing four parts water to one part salt by volume. Color the solution green using a few drops of green food coloring. 4. Show the students the three solutions and ask them to suggest a way to compare the masses of the three liquids. 5. Guide the discussion towards the realization that in order to compare the masses, equal volumes would have to be massed. Ask students to predict how the masses of the different liquids would be vary if the volume of each liquid is the same. Based on their predictions, have students formulate a hypothesis. 6. The topic of density as the relationship between mass and volume can now be introduced. EL8_2014 116 Part B: Student Procedure 1. On the electronic balance, mass the graduated cylinder and press "tare" to subtract the mass. If you are using a triple beam balance, mass the graduated cylinder and record this mass to the nearest 0.01g. Record the mass of the empty cylinder in the Data Table. 2. Pour 10 mL of the red liquid into the graduated cylinder. Use an eyedropper to get the exact amount of 10.0 mL. 3. To get a precise measurement, place the cylinder on a flat surface, bring your “eye” down to the level of the liquid, and read the bottom of the meniscus. 4. Determine the mass of the 10.0 mL by reading the electronic balance directly, or if using a triplebeam balance, record the total mass (cylinder + liquid) in the Data Table. Then subtract the mass of the empty graduated cylinder from the mass of the cylinder and sample of liquid. 5. Record the mass of the sample of liquid on the Data Table in the appropriate location, e.g. Red Liquid, volume of 10.0 mL. 6. Calculate the density of the liquid by dividing the mass by the volume (10 mL). 7. Record the density on the Data Table in the appropriate location, i.e. Red Liquid; volume of 10.0 mL. 8. Add another 10.0 mL to the cylinder. You should now have a total of 20.0 mL (10 mL + 10 mL). 9. Determine the mass of the 20.0 mL by reading the electronic balance directly, or if using a triplebeam balance, record the total mass CL (cylinder + liquid) record in the Data Table. 10. Then subtract the mass of the empty graduated cylinder (CE) from the mass of the cylinder and sample of liquid (CL). Record the mass of the sample of .liquid on the Data Table 11. Find the density again by dividing the mass by 20.0 mL and record it on the Data Table. 12. Keep adding 10.0 mL of the red liquid, recording the mass and calculating the density by dividing the mass by the amount of liquid in the cylinder until a total of 50.0 mL of the red liquid has been used. 13. Repeat the procedure for each of the other liquids, finding mass and density. 14. Graph mass (y-axis) vs. volume (x-axis) for each liquid on the graph paper provided. Use a different color for each of the liquid solutions. 15. Draw a line of “best-fit” for the points of each solution. Data Analysis Data Table for RED LIQUID Volume Mass of Empty (mL) Cylinder CE (g) Mass of Cylinder and Sample of Liquid CL (g) Mass of Sample of Liquid CL- CE (g) Density (g/mL) 10.0 20.0 30.0 40.0 50.0 EL8_2014 117 Data Table for BLUE LIQUID Volume (mL) Mass of Empty Cylinder CE (g) Mass of Cylinder and Sample of Liquid CL (g) Mass of Sample of Liquid CL- CE (g) Density (g/mL) Mass of Cylinder and Sample of Liquid CL (g) Mass of Sample of Liquid CL- CE (g) Density (g/mL) 10.0 20.0 30.0 40.0 50.0 Data Table for GREEN LIQUID Volume (mL) Mass of Empty Cylinder CE (g) 10.0 20.0 30.0 40.0 50.0 Analysis Questions: 1. Which variable, mass or volume, is considered the test variable (independent variable) in this experiment? 2. Which variable, mass or volume is considered the outcome variable (dependent variable) in this experiment? 3. As the volume increases, what happens to the mass of each sample? 4. Compare your density calculations for the red liquid. Should the density be the same in each instance? Explain your answer. Will this also be true for the blue and green liquids? 5. Analyze your data and determine which liquid is most dense and which one is least dense. Focusing on the mass and volume of each liquid. Identify what the relationship is between mass and volume in terms of density. EL8_2014 118 1. Predict what would happen to the liquids, if you carefully poured each liquid into a clear container. Write an explanation which differentiates the difference between each liquid of how and why they layered that way including the relationship of density to the location of each liquid. 2. In terms of density, differentiate between an object which floats in water and an object which sinks in water 3. Density of plain water is 1g/ml. What is the volume of a sample of water if the mass is 6.7g? Explain why this is so easy to figure out. 4. Show how one would set up a ratio to determine the mass of a substance with a density of 5.6g/mL and a volume of 3.7 mL. Then determine the mass. 5. Show how one would set up a ratio to determine the volume of a substance with a density of 2.6 g/mL and a mass of 5.5 g. Then determine the volume. 6. Based on the results of this lab, design an experiment demonstrating how unknown substances can be distinguished from one another by using their densities. Home Learning: Students will complete the Analysis Questions. Extensions: 1. Have students explore the density of objects with identical volumes, but different masses (use density cubes). Discover the relationship among mass, volume, and density. 2. Have students explore the density of different liquids and/or solutions, e.g. 5%, 10%, 15% saltwater solution. Discover the relationship between density and the solute concentration. EL8_2014 119 Literature Connection: “Archimedes and the King’s Crown” An ancient story tells about a Greek king, a gold crown and an amazing scientist named Archimedes. The king had ordered a solid golden crown made. When the court goldsmiths presented it to him, he asked Archimedes to test it to make sure it was pure gold. Archimedes knew that pure gold was very soft. He could bite a piece of it, and his teeth would leave a dent in it. (But he also knew that the king would be mad if he returned a dented crown. He couldn't use THAT test.) Archimedes also knew that if he took equal volumes of gold and water, the gold would weigh 23 times more than the water. He COULD use this test. (The problem was measuring the volume of the crown, an irregular object.). One night, while filling his tub, for a bath, Archimedes accidentally filled it to the very top. As he stepped into it, water spilled out over the top. The idea struck him, that if he collected the water, and measured it, he would know the volume of his body. HE COULD USE THIS TO MEASURE THE CROWN! In other words, the amount of displaced water in the bathtub was the same amount as the volume of his body. Archimedes was so excited that he jumped out of the tub. He ran outside and down the street yelling "Eureka! Eureka! (One of the few Greek words I know!) I found the answer!" www.sciencenet.org.uk/.../Chemistry/ StructBond/c00195b.html All this was fine except in his excitement, Archimedes had forgotten to put on his clothes. He was running down the street naked! Archimedes was able to get the volume of the crown and an equal volume of pure gold obtained, no doubt, from the King’s treasury. When he placed the two items into separate pans on a two-pan balance, well, I guess you can figure out the answer if I tell you that the goldsmith was put into jail! EL8_2014 120 DIFFERENTIATED INSTRUCTION: OPEN INQUIRY MASS, VOLUME, DENSITY Objectives/Purpose: Determine the physical properties of liquids. Demonstrate that regardless of the size of a sample, the density of a substance will always remain the same. Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured Demonstrate Achievement of the following Goals: Develop a problem statement based on the density of liquids that you would like to investigate. State your hypothesis. Design an experiment to test your hypothesis. Carry out the experiment you designed. Submit a completed lab report to your teacher. Use the “Claim, Evidence & Reasoning” rubric to defend your claims when writing your conclusion. EL8_2014 121 PRECIPITATING BUBBLES Review of the Scientific Method Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.N.1.1 Define a problem from the eighth grade curriculum using appropriate reference materials to support scientific understanding, plan and carry out scientific investigations of various types, such as systematic observations or experiments, identify variables, collect and organize data, interpret data in charts, tables, and graphics, analyze information, make predictions, and defend conclusions. (AA) SC.8.P.8.5 Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we encounter. (AA) (Also assesses SC.8.P.8.1, SC.8.P.8.6, SC.8.P.8.7, SC.8.P.8.8, and SC.8.P.8.9.) SC.8.P.9.2 Differentiate between physical changes and chemical changes. (AA) (Also assesses SC.8.P.9.1 and SC.8.P.9.3.) Background Information: (Reprinted from The Brain in Space: A Teacher’s Guide with Activities for Neuroscience, NASA, URL: http://science.nasa.gov/headlines/y2002/images/playingcatch/spacebrain.pdf) Scientists aim to gain knowledge and reach an understanding of the world around them. To achieve this goal, scientists must be curious, make observations, ask questions, and try to solve problems. Early scientists tended to draw conclusions from observations that were largely speculative (e.g., that the Earth was flat or that the Sun circled the Earth). By the mid-sixteenth century, some scientists began to realize that using a systematic approach to obtaining information and solving problems could obtain far more knowledge. This resulted in a process which we call the Scientific Method. Steps of a Scientific Method involving an experimental design Identify the problem. Collect information about the problem. Propose a hypothesis. Test the hypothesis by conducting experiments, making comparative observations, and collecting data. Evaluate the data collected through investigation. Draw conclusions based on data and determine whether to accept or reject the hypothesis. Communicate results and ask new questions. The problem is a statement of the question to be investigated. Observations and curiosity help to define exactly what problem should be investigated and what question(s) answered. Once a problem is defined, a scientist should collect as much information as possible about it by searching journals, books, and electronic information sources. This information will provide a basis for forming the hypothesis. A hypothesis is often considered to be an “educated guess.” The word “guess” is inappropriate, however, because a hypothesis should be based on information gathered. A hypothesis can be defined more accurately as a “proposed” answer to the problem, based upon background information either gathered through research or through experience. The hypothesis is then tested through experimentation and observation. The results of experimentation provide evidence that may or may not support the hypothesis. To be effective, experiments must be properly planned. The plan is called the procedure, which describes the things that actually will be done to perform the investigation. This is where decisions are made about EL8_2014 122 which variables will be tested and which will be kept constant, what to use as a control, how many samples to use, how large the sample sizes should be, safety precautions needed, and how many times to run the experiment. Many scientists investigate questions that cannot be answered directly through controlled experiments in laboratories. For example, scientists studying global warming, the AIDS epidemic, and losses of biodiversity must use comparative methods to examine differences that occur in the natural world. When developing the procedure for an experiment, consider the following: 1. Test only one variable at a time. A scientist wanting to find out “why trees shed their leaves in the fall” would have to consider the factors that affect trees, such as the type of tree, the amount of water they receive, the temperature, the length of daylight to which they are exposed, and the type of soil in which they are growing. These are the variables which can cause changes to occur in an experiment. To obtain reliable results, only one variable should be tested at a time. All others should be kept constant, whenever possible. If the scientist’s hypothesis states that shorter daylight hours cause trees to shed their leaves in the fall, trees of the same age should be tested. They should be placed in the same size pots with the same type of soil, given the same amount of water, and kept at the same temperature. The only thing changed should be the number of hours of light to which different groups of trees are exposed. Any variable that the experimenter chooses to change, such as the hours exposed to light, is referred to as the test variable (independent variable). The change in the experiment that happens as a result of the test variable, such as the length of time that it takes for the leaves to fall, is referred to as the outcome variable (dependent variable). 2. Use controls. The control is used for comparing the changes that occur when the variables are tested. If a number of young oak trees are placed in a greenhouse and exposed to 10 hours of light to simulate fall conditions, how will the scientist know if a loss of leaves is due to the amount of light? It could be due to the temperature that he/she chose or the amount of carbon dioxide in the air. To avoid such uncertainty, two identical experiments must be set up: one in which the trees are exposed to 10 hours of light and the other, the control, in which they are exposed to light for a longer period of time to simulate summer conditions. All factors for the control are exactly the same as for the test except for the variable being tested—the amount of light given to each tree. 3. Use several samples. Using a number of samples prevents errors due to differences among individuals being tested. Some trees are heartier than others. If only a few trees are tested, some may lose leaves for reasons that are not related to the amount of light. This will produce misleading results. Larger numbers of samples will provide more accurate results. 4. Always use appropriate safety measures. Safety measures to be followed vary according to the type of experiment being performed. For example, laboratory-based experiments frequently require that participants wear protective clothing and safety goggles and that dangerous volatile chemicals be used only under a vented fume hood. EL8_2014 123 5. Repeat the experiment several times. To make valid conclusions, the scientist must have reproducible results. Ideally, comparable results should be obtained every time the experiment is run. After the plan or procedure is complete, the experiment is run. It is essential that careful and accurate records be kept of all observations during an experiment. The recorded observations and the measurement comprise the data. It is always useful to present data in the form of charts, tables, or graphs, as these provide a visual way to analyze and interpret the results. When drawing graphs, the test variable (independent variable) is conventionally plotted on the horizontal axis, and the outcome variable (dependent variable) is plotted on the vertical axis. Analysis of data from the experiment allows the scientist to reach a conclusion. The scientist determines whether or not the data support the hypothesis and decides whether to accept or reject the hypothesis. The conclusion should provide an answer to the question asked in the problem. Even if the hypothesis is rejected, much information has been gained by performing the experiment. This information can be used to help develop a new hypothesis if the results repeatedly show that the original hypothesis is inappropriate. After performing many investigations on a particular problem over a period of time, a scientist may come up with an explanation for the problem, based on all the observations and conclusions made. This is called a theory. A Scientific Theory is an explanation, supported by data, of how or why some event took place in nature. MAJOR CONCEPTS FOR THE TEACHER Our exhaled breath contains carbon dioxide gas. The carbon dioxide we exhale reacts with calcium hydroxide in solution to form insoluble calcium carbonate and water.* Formation of calcium carbonate precipitate can be used as a test for the presence of carbon dioxide. If carbon dioxide continues to be bubbled into limewater (calcium hydroxide solution) after a period of time, the white precipitate disappears. The excess carbon dioxide forms carbonic acid in the water and the calcium carbonate reacts with the carbonic acid to form calcium ions and bicarbonate ions, which are soluble in water. ** Ca(OH)2 CO2 CaCO3 H2O H2CO3 Ca++ HCO3+ = calcium hydroxide = carbon dioxide = calcium carbonate = water = carbonic acid gas = calcium ion = bicarbonate ion Chemical Equations * Ca(OH)2 + CO2 CaCO3 + H2O ** CO2 + H2O + H2CO3 CaCO3 + H2CO3Ca++ 2HCO3+ This activity demonstrates the presence of carbon dioxide in exhaled air. In Activity 1, the teacher will blow through a straw into a solution of calcium hydroxide. The carbon dioxide in the exhaled air will combine with the calcium hydroxide to produce a white precipitate of calcium carbonate. In Part 2, EL8_2014 124 students will attempt to reproduce the experiment. They will not be able to do so because they will only have water as their unknown liquid. They should conclude that the teacher had a liquid other than plain water, resulting in a chemical reaction that changed one or more substances in the teacher’s original solution. The second part of this activity involves designing an experiment to test the hypotheses determined in the class discussion. It may be handled in different ways depending on the age of the students. Time Frame 30 minute teacher preparation 60 minutes / student activity MATERIALS 5 grams calcium hydroxide powder One liter of water Filter paper Filter funnel Flasks or small bottles Straws 25 mL or 50 mL graduated cylinder 125 mL Erlenmeyer flasks Test–tube rack Aluminum foil Stop watch Hot Plate Goggles Procedure: Part 1: Lab Prep The preparation of one liter of limewater (Should be prepared a day ahead of time): Teacher preparation 1. Add 10 grams calcium hydroxide Ca(OH)2 powder to 500 mL of water. 2. Cover and shake well. Calcium hydroxide is only slightly soluble in water and 5 grams will provide more solid than will dissolve. 3. Allow the suspension formed to settle for a few minutes. 4. To separate the limewater from the suspension, use the filter paper and filter–funnel apparatus to filter the suspension. 5. If the limewater filtrate is still slightly cloudy, filter for a second time, using a new filter paper. 6. Keep the limewater tightly closed when not in use, as it will react with carbon dioxide from the air and become cloudy. 7. The calcium hydroxide and water suspension can be stored in a large bottle, and the limewater filtered off when needed. 8. The filtered limewater can be stored in smaller bottles or flasks, 250 mL in volume, for use in class. Engage Part 1: 1. Read the following background information EL8_2014 125 2. 3. 4. 5. 6. 7. BACKGROUND Carbon dioxide comprises only 0.033 percent of Earth’s atmosphere, yet it is the principle inorganic source of carbon for living organisms. Carbon dioxide and water are the raw materials required by plants for the synthesis of sugars through photosynthesis. Organisms release carbon dioxide back into the atmosphere as a waste product of respiration and other cellular processes. Say to students: It is important for scientists to make careful observations, and you will practice doing the same in this activity. Keep a record of all of your observations. Everyone must wear safety goggles. Fill a 125 mL Erlenmeyer flask with 15 mL of teacher liquid (filtered limewater solution). Students record observations in notebook. Teacher will use a straw to bubble his/her breath into the liquid slowly for no more than 2 minutes. DO NOT blow vigorously as you do not want to spill the liquid! Be very careful not to allow any liquid to enter the mouth or eyes. Goggles are a must! Organize students into cooperative lab groups of 3 – 4 members. Assign each member a role (see Group Roles in the front of the packet). Data Analysis: Teacher Directed Part 1 1. Have group members discuss the following questions and place their answers on sticky notes. 2. Have one member of the group place their answers on the poster paper (one question/poster paper) provided by the teacher. Have another member read the group answers when called upon. 3. Questions for groups to answer: a. What gases are present in exhaled air? Carbon dioxide gas (nitrogen, water vapor, and small amounts of oxygen are also present.) b. What is the clear liquid? Limewater (calcium hydroxide) c. Why did a precipitate form? Why did the solution turn cloudy? There must have been a chemical reaction d. If a chemical reaction took place, what two ingredients do you think reacted? The limewater and the carbon dioxide e. How can we test for the presence of carbon dioxide? Bubble the gas into the clear limewater. f. What is a positive test for carbon dioxide? Limewater is a solution of calcium hydroxide. It chemically reacts with carbon dioxide to form solid calcium carbonate (chalk). 4. The responses from all groups will be discussed in class to ensure that all students understand the experiment. Data Analysis: Teacher Directed Part 2 1. Students will repeat the procedures demonstrated by the teacher. 2. Each student group is to measure 15 mL of student liquid (water) into a 125 mL Erlenmeyer flask, and record their observations in lab notebooks. 3. Using a straw, the assigned member of each group will bubble his/her breath into the liquid slowly for no more than 2 minutes. DO NOT blow vigorously to avoid spilling the liquid! 4. Observe the contents after blowing through the straws for approximately 1 – 2 minutes. 5. Record observations in lab notebook. These observations will be recorded as data. 6. When the teacher is convinced that class knows exactly what happened, he/she will say to the class, “Your teacher did the exact same experiment but got very different results!” His/her test tube has white precipitate. EL8_2014 126 7. The class now has a problem to solve: How can there be no white precipitate when the teacher performed the same experiment? 8. Groups will discuss what factors might affect the production of the precipitate (cloudy solution which will settle into a white solid and clear liquid in time). 9. Have groups propose a factor that might have affected the results. Possible answers might be: Time—how long exhaled air was bubbled into the solution. Adults vs. teenagers Rate of bubbling Light vs. dark Temperature of the liquideither hotter or colder Different substance The factors identified are known as variables. 10. Each group will be assigned at least one of the variables to test. 11. Use the following questions to guide the groups in the development of their group hypotheses and experimental design: • Does the hypothesis offer an answer to the problem? Yes, it does. The problem was, “Why was there a white precipitate when the teacher performed the experiment?” The hypothesis states that the teacher may have (choose a variable). • Does the experiment have a control? Yes. The control is the average length of time that the students exhaled into the liquid (possibly about one minute). • Which materials are needed? Are the materials readily available? • What conditions are being kept constant? The conditions kept constant are the temperature of the liquid, the size of the straws, the rate of bubbling into the liquid and the amount of liquid used for each test (there may be human error here – may not blow at same rate consistently). • What is the test variable (independent variable) being tested? This is the variable that the experimenter chooses to change. • What is the outcome variable (dependent variable) being measured? The outcome variable (dependent variable) is the presence or absence of precipitate present after exhaling into the container. • How will each group present its data? Presentation format will vary 12. Each group must submit to the teacher prior to any experimentation a proposed hypothesis; a draft procedure (which may be modified as students work through the experiment); a draft data table Procedure: Part 3 1. Groups will be provided with the needed materials to perform their experiments, collect data, and draw conclusions. 2. Each group must turn in a completed Laboratory Report. 3. A post-experiment class discussion may be conducted to review the conclusions made by each group. 4. Compare the experiments performed by each group of students. For each experiment designed, discuss the variable tested, the control, the factors kept constant (controlled variables), and the results obtained. Note that the amount of limewater used and the size of the straws and flasks should be the same for each experiment. A chart, such as the one below, can be developed on an overhead projector. EL8_2014 127 5. Add any other variables tested to the chart as necessary. 6. From the class observations, it can be concluded that only the length of time affects the amount of precipitate formed. However, results have also varied based upon how vigorous the blowing was, i.e. amount of carbon dioxide introduced. 7. At this point, explain that excess carbon dioxide bubbled into limewater forms carbonic acid, which dissolves the precipitate of calcium carbonate. Place balanced chemical equation on the board. 8. The use of a Scientific Method, specifically an experimental design to systematically test different hypotheses will enable the students to determine which hypothesis is correct in answering a problem. Evaluation: 1. You will be evaluated according to the amount of effort expanded, your specific job performance, your participation within the team, and on the final product—the laboratory write-up. 2. Your group’s experiment should be evaluated based on the appropriateness of the design you initiated to test the group’s hypothesis (not whether the group actually found the “correct” solution. 3. Students will be asked to test the hypothesis that “the length of time that air was blown into the solution” caused the teacher’s results to be different. Each student in a group of four will use the same size tube and the same amount of lime water, run the experiment at the same temperature, use the same size straws, and attempt to bubble at the same rate. Students should estimate how long they exhaled into their liquid the first day. This could be the control time. One student in each group will blow into his/her tube for the control time. Each of the remaining students in the group should increase the control time by two to four minutes. 4. Explain why there was no change in the student liquid when carbon dioxide was exhaled into the liquid. Home Learning: 1. Work on designing and writing-up an experimental design for completion of Part 2. 2. Work on the completion of the laboratory write-up, which may include data analysis, graphing, and drawing conclusions after completion of Part 3. 3. Discussion and provide examples of chemical changes where new substances are formed as a result of atoms combining – some students may discuss that this is a result of electron bonds forming. Extensions: 1. Have each group perform four more different experiments, to test several variables. 2. Do not share the final chemical equation with students. Additionally, challenge them to find the correct reaction mechanism. EL8_2014 128 Student PRECIPITATING BUBBLES Florida Next Generation Sunshine State Standards Benchmark(s): SC.8.N.1.1: Scientific Investigations SC.8.P.8.5: Combining Atoms SC.8.P.9.2: Chemical and Physical Changes BACKGROUND Carbon dioxide comprises only 0.033 percent of Earth’s atmosphere, yet it is the principle inorganic source of carbon for living organisms. Carbon dioxide and water are the raw materials required by plants for the synthesis of sugars through photosynthesis. Organisms release carbon dioxide back into the atmosphere as a waste product of respiration and other cellular processes. Part 1: Teacher Demonstration Observations: What did you observe in the teacher demonstration? Questions: a. What gases are present in exhaled air? b. What was the clear liquid in the initial demonstration? c. Why did a precipitate form? Why did the solution turn cloudy? d. If a chemical reaction took place, what two ingredients do you think reacted? e. How can we test for the presence of carbon dioxide? f. What is a positive test for carbon dioxide? EL8_2014 129 Part 2: Repeat the procedures demonstrated by the teacher. Procedures: 13. Each student group is to measure 15 mL of student liquid (water) into a 125 mL Erlenmeyer flask, and record their observations in lab notebooks. 14. Using a straw, the assigned member of each group will bubble his/her breath into the liquid slowly for no more than 2 minutes. DO NOT blow vigorously to avoid spilling the liquid! 15. Observe the contents after blowing through the straws for approximately 1 – 2 minutes. 16. Record observations in lab notebook. These observations will be recorded as data. Observations: Questions: 1. Was there a problem with the results? Explain. 2. How can there be no white precipitate when the teacher performed the same experiment? 3. What factors might affect the production of the precipitate (cloudy solution which will settle into a white solid and clear liquid in time)? ** The factors identified are known as variables. Each group will be assigned at least one of the variables to test. ** Group Variable: ___________________________________________________________________________________ EL8_2014 130 Part 3: Design an Experiment to Test Your Variable Problem Statement: The question you want to answer Hypothesis: “If (this is changed) then (this will happen) because...” Test Variable: Factor being tested Outcome Variable: Factor being measured Control Group: Used as a comparison Constant Conditions: Purposefully kept the same Materials Needed: Procedures: Specific steps you will take to test the hypothesis. Be Specific! Data: Observations, Charts, Tables, and/or Graphs EL8_2014 131 Conclusion: Claim: Make a CLAIM based on what you observed in the experiment you performed today Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. EL8_2014 132 DIFFERENTIATED INSTRUCTION: OPEN INQUIRY PRECIPITATING BUBBLES Objectives/Purpose: Demonstrate that organisms release carbon dioxide back into the atmosphere as a waste product of respiration and other cellular processes. Design an investigation based on observations Understand that accurate record keeping, openness, and replication are essential to maintaining an investigator's credibility with other scientists and society. Demonstrate Achievement of the following Goals: After completing the “Precipitating Bubbles Essential Lab”, develop a problem statement based on an unanswered question you have that you would like to investigate. State your hypothesis. Design an experiment to test your hypothesis. Carry out the experiment you designed. Submit a completed lab report to your teacher. Use the “Claim, Evidence & Reasoning” rubric to defend your claims when writing your conclusion. EL8_2014 133 GREENHOUSE GASES IN A BOTTLE Next Generation Sunshine State Standard Benchmark: SC.8.L.18.3 Construct a scientific model of the carbon cycle to show how matter and energy are continuously transferred within and between organisms and their physical environment. SC.8.L.18.4 Cite evidence that living systems follow the Laws of Conservation of Mass and Energy. (AA) Background Information for the teacher: Particles suspended in our atmosphere (aerosols) can absorb more sunlight or they can reflect the Sun's energy back into space. The Earth's temperature would be much colder without the CO2 in our atmosphere we have naturally. When we add more, the Earth warms up. The effects of atmospheric CO2 and aerosols on our planet's temperature are measurable with simple tools anyone can use. Greenhouse gases are carbon dioxide, methane, nitrous oxide, ozone (in the lower atmosphere), water vapor and CFCs. One greenhouse gas that has been increasing in the past 50 years is carbon dioxide. Loss of rainforests that take in carbon dioxide and the burning of fossil fuels by cars, factories and plants that releases carbon dioxide is part of the causes. CLAIM-EVIDENCE-REASONING-A persistent question with regard to the greenhouse effect is, "Why does the light energy from the Sun pass through the greenhouse gases in the layers of the atmosphere but are trapped once the infrared light returns to space after reflected off Earth?” Materials: Funnel Filter paper for measuring baking soda Graduated Cylinder Timer/Stopwatch Four: 500 mL clear water bottles with the label removed Identical thermometers for each soda bottle Duct tape Source of carbon dioxide (CO2)-vinegar and baking soda Modeling clay Measuring spoons Balloons 125 mL Erlenmeyer 500 mL of room temperature water Optional Heat Lamps Triple-Beam Balance Flask Engage: Read or write on the board "Why does the light energy from the Sun pass through the greenhouse gases unhindered and the infrared energy radiated from the Earth is absorbed?" Explain how a greenhouse is able to maintain a temperature at which plants are able to grow even though the temperature outside the greenhouse sometimes will not support plant life. Relate a greenhouse to how the Earth’s atmosphere traps heat. Identify the gases in the atmosphere that “act” like the glass in a greenhouse. Optional: Studyjams-Carbon Cycle, BBC-Carbon Cycle Explore: Teacher Preparation: 1. Drill the caps of the bottles to the same diameter as your thermometer. Place the thermometers through the holes in the caps several inches. Use the modeling clay to hold the thermometers in place and seal the hole. 2. Number bottles #1, #2, #3, and #4. EL8_2014 134 Name:___________________________________________ Period: _______Date: ____________ Procedure: For your source of carbon dioxide, use the following methods quickly to ensure CO2 is captured: Bottles #1 and #2 - Carefully mix 10 grams of baking soda and 50 mL vinegar in a flask. Cover with balloon to capture the CO2. Add 50 mL water to bottle #1. Release the CO2 into bottle #1 and cover with cap quickly. Repeat the procedure for the bottle #2. Bottles #3 and #4 – Pour10 grams of baking soda into each bottle. Now add 50 mL of water to each bottle. 3. Place the caps with thermometers onto the tops of the bottles. 4. Place bottles in sunlight. Make sure they receive the same amount of sun. NOTE: a heat lamp may be substituted for the sun, but you must be very careful to place the bottles exactly the same distance from the lamp. 5. Shade the thermometers by putting a strip of opaque tape on the outside of the bottles. The tape must be the same length on all bottles. 6. Measure the temperature of the bottles over time. Record the temperature of each bottle every five minutes for a half hour Data Table Dry Elapsed Time in minutes Initial 5 10 15 20 25 30 Bottle 1 Wet Bottle 2 Bottle 3 Bottle 4 Explain: Conclusion 1. Interpret the graph and identify a trend for the change in temperature for each container during the experiment? Did both jars show the same change in temperature? Calculate the change in temperature for each jar. 2. Did your results support your hypothesis? 3. Explain why the temperature of the covered jar showed an increase in temperature. What part of this setup contributed to the increase in temperature? 4. Explain how the covered jar setup represents an experimental model of the influences of the greenhouse effect on the temperature of the Earth’s atmosphere. Identify what the light bulb and plastic wrap represent in this experimental model. 5. Identify the tested (independent), outcome (dependent) and controlled (constant) variables in this experiment. 6. In this experiment we only tested each setup one time (20 minute interval); explain why this will affect the validity of the data. How can we change this experiment so the data will be more valid? 7. Based on what you learned in this activity, can you connect this knowledge to the environmental issue of the dangers of the greenhouse effect? Explain 8. Think about what humans do that increases the amount of greenhouse gases released into the atmosphere and develop a list of ways that we can reduce the level of these gases. EL8_2014 135 Elaborate: Claim: Make a CLAIM based on what you observed in the experiment you performed today. Evidence: Support your claim using EVIDENCE you collected in your experiment. Reasoning: Use science concepts to provide REASONING for why the evidence you presented supports your claim. Optional Extensions: 1. Activity # 1. Students may want to continue the experiment and record the two temperatures every day at the same time for a week. Graph the data and discuss how the temperatures fluctuate from day to day. 2. Activity # 2. Green House Gases. There is no scientific dispute about the presence of "greenhouse gases" (including carbon dioxide--CO2) in the Earth’s atmosphere that function to trap heat from the Sun. There is also no dispute that the amount of CO2 in the atmosphere has increased 25%. Does this mean that global warming is occurring? Nobody knows for certain, but many atmospheric scientists are becoming concerned about the increasing amount of CO2 in the atmosphere. What does this mean to you? Despite the uncertainties, if global warming does occur (or if it has already begun), it will profoundly affect human societies. Global warming may result in severe droughts, reducing crop production necessary to feed billions of people. Rising sea levels will threaten beaches, coastal cities, and people. The migration of millions of people would strain economic, health, and social services. Conflicts over remaining resources could escalate. Wildlife habitat will be destroyed, with countless species facing extinction. With the potential devastating effects of global warming, it is reasonable and prudent to examine alternatives to fossil fuels to decrease the amount of CO2 in the atmosphere. The transportation sector is one area that can, generally speaking, use alternative methods of fuel, since there are already a variety of alternate fuels available. The good news is that this transition can be done relatively easily, cheaply, and painlessly. EL8_2014 136 STUDENT HANDOUT DIFFERENTIATED INSTRUCTION: OPEN INQUIRY MODELING THE GREENHOUSE EFFECT Objectives/Purpose: In this investigation students will: Create models of Earth to demonstrate the greenhouse effect. Relate a greenhouse to how the Earth’s atmosphere traps heat. Identify the gases in the atmosphere that “act” like the glass in a greenhouse. Demonstrate Achievement of the following Goals: Develop a problem statement based on the concept of the “greenhouse effect” that you would like to investigate. Create a model to demonstrate the concept of the “greenhouse effect” that you would like to investigate. How does the model demonstrate the concept that you investigated? Remember to use the “Claim, Evidence & Reasoning” rubric to defend your claims. EL8_2014 137 IMAGINARY ALIEN LIFE FORMS Adapted from Mars Critters http://ares.jsc.nasa.gov/ares/education/program/Data/marsCritters.pdf and Solar System Activities: Search for a Habitable Planet http://solarsystem.nasa.gov/educ/docs/modelingsolarsystem.pdf Next Generation Sunshine State Standards Benchmark: SC.8.E.5.7 Compare and contrast the properties of objects in the Solar System including the Sun, planets, and moons to those of Earth, such as gravitational force, distance from the Sun, speed, movement, temperature, and atmospheric conditions. (AA) (Also assesses SC.8.E.5.4 and SC.8.E.5.8.); SC.7.L.15.2 Explore the scientific theory of evolution by recognizing and explaining ways in which genetic variation and environmental factors contribute to evolution by natural selection and diversity of organisms. (AA) (Also assesses SC.7.L.15.1 and SC.7.L.15.3.) SC.7.L.16.2 Determine the probabilities for genotype and phenotype combinations using Punnett Squares and pedigrees. About This Activity In groups or as individuals, students will use their knowledge of Mars and living organisms to construct a model of a plant or animal that has the critical features for survival on Mars. This is a “what if” type of activity that encourages the students to apply knowledge. They will attempt to answer the question: What would an organism need to be like in order to live in the harsh Mars environment? Objectives Students will: • draw logical conclusions about conditions on Mars. • predict the type of organism that might survive on Mars. • use a Punnett Square to predict offspring genotype and phenotype • construct a model of a possible Martian life form. • write a description of the life form and its living conditions focusing on necessary structural adaptations for survival. Background To construct a critter model, students must know about the environment with extremes in temperature. The atmosphere is much thinner than the Earth’s; therefore, special adaptations would be necessary to handle the constant radiation on the surface of Mars. Also the dominant gas in the Mars atmosphere is carbon dioxide with very little oxygen. The gravitational pull is just over 1/3rd (0.38) of Earth’s. In addition, Mars has very strong winds causing tremendous dust storms. Another requirement for life is food—there are no plants or animals on the surface of Mars to serve as food! Scientists are finding organisms on Earth that live in extreme conditions previously thought not able to support life. Some of these extreme environments include: the harsh, dry, cold valleys of Antarctica, the ocean depths with high pressures and no Sunlight, and deep rock formations where organisms have no contact with organic material or Sunlight from the surface. EL8_2014 138 Vocabulary Ecology, adaptations, gravity, geology, atmosphere, radiation exposure, weather, environment, genotype, phenotype Part 1 Materials paper (construction, tag board, bulletin board, etc.) colored pencils glue items to decorate critter (rice, macaroni, glitter, cereal, candy, yarn, string, beads, etc.) pictures of living organisms from Earth Student Sheet, Mars Critters Student Sheet - Activity 1, If You Went to Mars Mars Fact Sheet (pg. 56) Procedure Advanced Preparation Gather materials. Set up various art supplies at each table for either individual work or small group work. This activity may be used as a homework project. Review the “If You Went to Mars” sheet, Mars Fact Sheet, and the background provided above along with the research conducted in the Martian Sun-Times activity or other desired research. Classroom Procedure 1. Ask students to work in groups to construct a model of an animal or plant that has features that might allow it to live on or near the surface of Mars. 2. Have them consider all the special adaptations they see in animals and plants here on Earth. 3. They must use their knowledge of conditions on Mars, consulting the Mars Fact Sheet, If You Went to Mars, and other resources such as web pages if necessary. Some key words for a web search might be “life in space” or “extremophile” (organisms living in extreme environments). 4. They must identify a specific set of conditions under which this organism might live. Encourage the students to use creativity and imagination in their descriptions and models. 5. If this is assigned as homework, provide each student with a set of rules and a grading sheet, or read the rules and grading criteria aloud and post a copy. 6. Review the information already learned about Mars in previous lessons. 7. Remind the students that there are no wrong critters as long as the grading criteria are followed. 8. Include a scale with each living organism. 9. Students select two different organisms that will mate. 10. Revisit/Introduce Genetics:: Select one trait, the height of the “Mars Critter,” and generate a Punnett Square to predict the genotype (genetic make-up) and phenotype (physical characteristics) of the offspring that the two organisms would produce, if mated. Students will learn more about this in upcoming topics. For simplicity – tell students that the height trait will have a paired allele, each parent giving one possible allele to the offspring and tall is dominant and expressed in the offspring when present. Complete a sample Punnett Square, as a reminder. Advanced students may explore incomplete dominance. EL8_2014 139 As an extension, mate offspring and/or generate Punnett Squares for other characteristics. Genotype TT (dominant tall) tt (recessive short) Tt (mixed hybrid) Phenotype Tall Short Tall Teacher Guide Source: www.exploringnature.org/db/detail.php?dbID=22&detID=2290 EL8_2014 140 Description and Questions Use another page if more space is needed. 1. The critter’s name: 2. Describe the habitat and climate in which your critter lives. 3. How does it move? Include both the form and method of locomotion. (For example: The miniature Mars Gopher leaps on powerful hind legs.) 4. What does it eat or use as nutrients? Is it herbivorous, carnivorous, omnivorous, other? What is its main food and how does it acquire this food? 5. What other creatures does it prey on, if any: How does it defend itself against predators? 6. How does your creature cope with Mars’ extreme cold, unfiltered solar radiation, and other environmental factors? 7. Suppose two Mar’s critters mated. One was Tall and the other was short. Using a Punnett Square, predict the offspring’s possible heredity of the tall gene. Each parent has two alleles for the height gene. Dad is homozygous tall (TT) and mom is short (tt). Predict the genotype (genetic make-up) and phenotype (physical characteristics) for the offspring Dad Genotype: _____% TT ____% tt ____% Tt Phenotype: ______% Tall ______% short Genotype TT (homozygous tall) tt (homozygous short) Tt (heterozygous) EL8_2014 Phenotype Tall Short Tall Offspring Mom 141 Student MARS FACT SHEET If You Went to Mars From: “Guide to the Solar System.” By the University of Texas, McDonald Observatory Mars is more like Earth than any other planet in our solar system but is still very different. You would have to wear a space suit to provide air and to protect you from the Sun’s rays because the planet’s thin atmosphere does not block harmful solar radiation. Your space suit would also protect you from the bitter cold, temperatures on Mars rarely climb above freezing, and they can plummet to -129oC (200 degrees below zero Fahrenheit). You would need to bring water with you, although if you brought the proper equipment, you could probably get some Martian water from the air or the ground. The Martian surface is dusty and red, and huge duststorms occasionally sweep over the plains, darkening the entire planet for days. Instead of a blue sky, a dusty pink sky would hang over you. West Rim of Endeavour Crater on Mars Image Credit: NASA/JPL-Caltech/Cornell/ASU http://www.nasa.gov/mission_pages/mer/multimedia/gallery/pia11507.html EL8_2014 142 Fourth planet from the Sun Distance from the Sun: Minimum: 206,000,000 kilometers Average: 228,000,000 kilometers (1.52 times as far as Earth) Maximum: 249,000,000 kilometers Eccentricity of Orbit: 0.093 vs. 0.017 for Earth (0.00 is a perfectly circular orbit) Distance from Earth: Minimum: 56,000.000 kilometers Maximum: 399,000,000 kilometers Year: 1.88 Earth years - 669.3 Mars days (sols) – 686.7 Earth days Day: 24.6 Earth hours Tilt of Rotation Axis: Size: 25.2o vs. 23.5o for Earth Diameter: 6794 kilometers vs 12,76 kilometers for Earth Surface Gravity: 0.38 9 or ~ 1/3) Earth’s gravity Mass: 6.4 x 1026 grams vs. 59.8 x 1026 grams for Earth Density: 3.9 grams/mL vs. 5.5 grams/mL for Earth Surface Temperature: Cold Global extremes: -125oC (-190oF) to 25oC (75oF) Average at Viking 1 site high 010oC (15oF); low -90oC (-135oF) Atmosphere: Thin, un-breathable Surface pressure: ~6 millibars, or about 1/200th of Earth’s -Contains 95% carbon dioxide, 3% nitrogen, 1.5%argon, ~0.03% water (varies with season), no oxygen. (Earth has 78% nitrogen, 21% oxygen, 1% argon, 0.03% carbon dioxide.) Dusty, which makes the sky pinkish. Planet-wide dust storms black out the sky. Surface: Color: Rust red Ancient landscapes dominated by impact craters Largest volcano in the solar system (Olympus Mons) Largest canyon in the solar system (Valles Marineris) Ancient river channels Some rocks are basalt (dark lava rocks), most others unknown Dust is reddish, rusty, like soil formed from volcanic rock Moons Phobos (“Fear”), 21 kilometers diameter Deimos (“Panic”), 12 kilometers diameter EL8_2014 143 Part 2: Search for a Habitable Planet Next Generation Sunshine State Standards: SC.8.E.5.3 Distinguish the hierarchical relationships between planets and other astronomical bodies relative to solar system, galaxy, and universe, including distance, size, and composition. (AA)(Also assesses SC.8.E.5.1 and SC.8.E.5.2.) SC.8.E.5.7 Compare and contrast the properties of objects in the Solar System including the Sun, planets, and moons to those of Earth, such as gravitational force, distance from the Sun, speed, movement, temperature, and atmospheric conditions. (AA)(Also assesses SC.8.E.5.4 and SC.8.E.5.8.) Objective: This lesson focuses on characteristics of planets that make them habitable. Living creatures need food to eat, gas to breathe, and a surface that provides a comfortable temperature, gravity, and place to move around. These requirements are related to what the planet’s surface and atmosphere are made of, and how large (gravity) and close to the Sun (temperature) the planet is located. The inner planets are small (low gravity), relatively warm, and made of solid rock. Some of them have atmospheres. The outer planets are large (high gravity), cold, and made of gaseous and liquid hydrogen and helium. A creature that might be comfortable on a gas giant would not be comfortable on a small rocky planet and vise versa. Vocabulary: habitable, life requirements, planet characteristics, surface and atmospheric composition (chemical examples) Time Required: One to two 45 minute class periods Materials: Creature Cards Solar System Images and Script Planet Characteristics Table Students will define the life requirements of a variety of creatures and learn that these relate to measurable characteristics of planets the creatures might inhabit. By evaluating these characteristics, students discover that Earth is the only natural home for us in our solar system and that Mars is the next most likely home for life as we know it. EL8_2014 144 Procedures Activity 1. Define Habitability and Design Creatures This lesson has students take the places of extraterrestrial creatures exploring our solar system in search of new homes. They define creature life requirements and relate them to planet characteristics in order to choose homes. Several of these creatures have life requirements quite unlike life as we know it, where water and carbon are essential, and some are downright impossible. The goals here are not to study biochemistry, but habitability of planets. Bizarre creatures had to be invented for them to find homes on some of the planets in our solar system. Another goal is to encourage creativity and teamwork in designing creatures and selecting planets. This activity is one that is outside of the box. ENGAGE 1. Set the stage by reading introduction: We are space travelers from a distant star system. The crew of our spaceship includes six different types of creatures who live on different planets in that star system. Our star is expanding and getting very hot. Our home planets are heating up and soon we will need new places to live. It is our mission to find habitable planets for our six different types of creatures with different life requirements. In all we need to find new homes for five billion inhabitants. First we need to know what makes a planet habitable so we can set up probes to measure the characteristics of various planets. The different requirements for life can be related to measurable planetary characteristics. What do creatures require to live? EXPLORE 2. Brainstorm on requirements and characteristics. Lead the students in producing a table similar to the one below. Encourage free-thinking, there aren't specific right answers, but lead students to the following topics, among others. Life requirements food to eat gas to breathe comfortable temperature ability to move Planet characteristics surface & atmosphere composition atmosphere composition temperature range surface type (solid, liquid, gas) gravity size 3. Ask students what kinds of probes might be used to measure these characteristics. Answers may range from general to specific and may be based on science fiction. Examples may include cameras, radar, thermometers, and devises to measure magnetics, altitude, and light in all wavelengths from radio waves, through infrared, ultraviolet, and X-ray to gamma-ray. [Secondary school classes might do one of the excellent activities on the electromagnetic spectrum or activities related to solar system missions.] 4. Divide students into six or more teams (more than one group can design the same creature). Explain that each team represents one of the six different types of creatures on our mission. Today we will make models of creatures having specific life requirements. Later we will collect data on a new planetary system in order to search for new homes. 5. Distribute one creature card to each team. Each card contains the information on a single line A-F below. Tell students that each team is supposed to create a creature that fits the characteristics on their creature card. Students may select art supplies (or drawing supplies) and should be able to complete their creatures in approximately 15-20 minutes. Students will name their creature ambassador and be ready to introduce it to the class. Encourage teamwork and creativity. EL8_2014 145 [Teacher, you may get questions on some of the food or gases. Handle these as they come, but do not provide this vocabulary ahead of time unless it comes up during brainstorming. Simply explain that they are various chemical elements or compounds. They are needed only for matching with planetary characteristics and should not be tested vocabulary.] 6. Ask each team to introduce their creature ambassador and to explain their creature's needs and any specific features of the model. This will take longer than you expect because students really get involved with their creatures. Creature A B C D E F Food helium rock carbon methane water carbon Breathes hydrogen carbon dioxide oxygen hydrogen carbon dioxide oxygen Motion flies flies walks swims walks swims Temperature cold hot moderate cold moderate moderate Assessment: Evaluate team presentations and collect descriptions of how their creature meets its life requirements. EXPLAIN Activity 2. Tour solar system and evaluate for habitability 1. Prepare students for solar system tour. Tell students that they will have to take notes on the planets to report back later. Students will work in the same teams as when they made creatures. The grade level/ability will determine how the teacher structures the information gathering. Each team may record the information on all planets or on just one or two planets. Young students may simply compare planet characteristics to those on their creature cards and check off boxes of matching characteristics on the planet chart. 2. Distribute copies of the blank planet characteristics chart or put it on the blackboard/overhead. Show slides/photos of the planets and read the text provided below. For elementary students, exclude the data in parentheses. For secondary students, include the data. As you tour the planets, it may be necessary to repeat each section twice for younger students to get enough information to report. 3. Compile information on overhead or blackboard planet characteristics chart as teams report data they recorded on planet (size, surface type, composition, atmosphere and temperature). Attached table gives suggested answers. Students will probably be able to name the planets, but this is not a test. Alternatively, each student could fill in a chart to allow evaluation of listening skills. Also, students could work cooperatively to complete one chart per team. 4. Have teams compare the characteristics chart on the planets with the creature requirements on their creature card. Decide which planets (if any) would be suitable homes for their creature. Report their choices orally and explain, if necessary. Tabulate on the blackboard. Creature Planet(s) A 4, 5 (Saturn and Jupiter), but also 2,3 (Neptune and Uranus) B 8 (Venus) C, F 7 (Earth) D 2,3 (Neptune and Uranus) E 6 (Mars) No creatures can live on planets 1 or 9 (Mercury or Pluto) EL8_2014 146 5. Ask students to create a finale or read the finale below. Now that the creatures have evaluated habitable planets we will send down spaceships to check out the surfaces in detail. Creatures A, B, D and E find uninhabited planets that are just suited to their needs. They decide to settle on their chosen planets. Creatures C and F are both interested in the same planet. Creature F finds the salt water to be a perfect home for it, while creature C finds the land to be overpopulated and polluted. They decide that there isn't room for one billion more inhabitants and decide to look for a habitable planet in another solar system. Assessment: Collect Planet Characteristics tables and compare with the suggested answers above. Do not require a perfect match, but allow students to think critically and creatively. Allow adaptations of the environment (such as turning water into hydrogen and oxygen) and other reasonable modifications. EVALUATE Writing assignment: Ask students to write a paragraph explaining why the planet they found will or will not be suitable for their creature. The paragraph could be in the form of a news report to be sent back to their dying solar system. EL8_2014 147 CREATURE CARDS We are space travelers from a distant star system. The crew of our spaceship includes six different types of creatures who live on different planets in that star system. Our star is expanding and getting very hot. Our home planets are heating up and soon we will need new places to live. It is our mission to find habitable planets for our six different types of creatures with different life requirements. In all we need to find new homes for five billion inhabitants. Your task 1) Design a creature that fits the following needs for life. 2) Give it a name. and 3) Introduce it to the class and explain how it meets its needs for life. Creature A Food Helium Breathes Motion Hydrogen Flies Temperature Cold We are space travelers from a distant star system. The crew of our spaceship includes six different types of creatures who live on different planets in that star system. Our star is expanding and getting very hot. Our home planets are heating up and soon we will need new places to live. It is our mission to find habitable planets for our six different types of creatures with different life requirements. In all we need to find new homes for five billion inhabitants. Your task 1) Design a creature that fits the following needs for life. 2) Give it a name. and 3) Introduce it to the class and explain how it meets its needs for life. Creature B Food Breathes Motion Temperature EL8_2014 Rock Carbon dioxide Flies Hot 148 We are space travelers from a distant star system. The crew of our spaceship includes six different types of creatures who live on different planets in that star system. Our star is expanding and getting very hot. Our home planets are heating up and soon we will need new places to live. It is our mission to find habitable planets for our six different types of creatures with different life requirements. In all we need to find new homes for five billion inhabitants. Your task 1) Design a creature that fits the following needs for life. 2) Give it a name. and 3) Introduce it to the class and explain how it meets its needs for life. Creature C Food Breathes Motion Temperature Carbon Oxygen Walks Moderate We are space travelers from a distant star system. The crew of our spaceship includes six different types of creatures who live on different planets in that star system. Our star is expanding and getting very hot. Our home planets are heating up and soon we will need new places to live. It is our mission to find habitable planets for our six different types of creatures with different life requirements. In all we need to find new homes for five billion inhabitants. Your task 1) Design a creature that fits the following needs for life. 2) Give it a name. and 3) Introduce it to the class and explain how it meets its needs for life. Creature D Food Breathes Motion Temperature EL8_2014 Methane Hydrogen Swims Cold 149 We are space travelers from a distant star system. The crew of our spaceship includes six different types of creatures who live on different planets in that star system. Our star is expanding and getting very hot. Our home planets are heating up and soon we will need new places to live. It is our mission to find habitable planets for our six different types of creatures with different life requirements. In all we need to find new homes for five billion inhabitants. Your task 1) Design a creature that fits the following needs for life. 2) Give it a name. and 3) Introduce it to the class and explain how it meets its needs for life. Creature E Food Breathes Motion Temperature Water Carbon Dioxide Walks Moderate We are space travelers from a distant star system. The crew of our spaceship includes six different types of creatures who live on different planets in that star system. Our star is expanding and getting very hot. Our home planets are heating up and soon we will need new places to live. It is our mission to find habitable planets for our six different types of creatures with different life requirements. In all we need to find new homes for five billion inhabitants. Your task 1) Design a creature that fits the following needs for life. 2) Give it a name. and 3) Introduce it to the class and explain how it meets its needs for life. Creature F Food Breathes Motion Temperature EL8_2014 Carbon Oxygen Swims Cold 150 Search for a Habitable Planet Solar System Images and Script Today we are traveling through an outer section of the Milky Way galaxy. There are many, many stars. We are approaching a medium-sized star, the type that often has habitable planets. As we draw closer we see that there are nine planets orbiting this star. We will tour this planetary system and use our probes to measure planet characteristics in our search for a habitable planet. Record this information about your planet then when we have completed our tour we will collect all our results. We will evaluate our results to look for a new place to live. We will now tour this new planetary system, starting from the outside and going toward the star: We are approaching the first planet. The first “planet” is tiny (2350km). In fact, it was downgraded from a planet to a dwarf planet in 2006 mainly because it orbits around the Sun in “zones of similar objects that can cross its path.” It is made of rock and methane ice. It has almost no atmosphere (just a trace of methane) and is very cold (-230oC). The second planet is a medium large (49,500km) and made of liquid hydrogen and helium. It has a thick atmosphere of hydrogen, helium and methane. It is very cold (-220 oC). The third planet is very similar to the 2nd except that it has a small ring system. It is medium large (51,000 km) and made of liquid hydrogen and helium. It also has a thick atmosphere of hydrogen, helium and methane and is very cold (-210 oC). The fourth planet is large (120,500 km) and has an extraordinary ring system. It has no solid surface, but is a giant mass of hydrogen and helium gas outside and liquid hydrogen inside. It is cold (-180 oC). EL8_2014 151 Search for a Habitable Planet Solar System Images and Script The fifth planet is the largest (143,000 km) in this planetary system. Like the fourth, it is a gas giant made of hydrogen and helium with no solid surface. It is also cold (-150oC) in the upper atmosphere, but increases in temperature and pressure and becomes liquid in the interior. The sixth planet is small (6786 km) and rock. There is some water ice in polar regions and a thin atmosphere of carbon dioxide. The temperature is moderate (-23oC). The seventh planet is medium small (12, 750 km). The surface is made of liquid water and rock with some carbon compounds. The atmosphere is mostly nitrogen and oxygen with some carbon dioxide and water vapor. The temperature is moderate (21oC). The eighth planet is also medium small (12,100 km). The atmosphere of carbon dioxide is so thick that we can’t see the rocky surface beneath it, but need our radar probes. The temperature is very hot (480oC). The ninth planet is tiny (4880 km) and rocky. It has almost no atmosphere (just a hint of helium). Temperatures are generally hot, but extreme variable, ranging from -180oC on the space-facing side to 400oC on the star-facing side. We have now finished our tour and it’s time to compile all of our data. Each team will report its results and we will make a comparison chart. EL8_2014 152 PLANET CHARACTERISTICS (Teacher Key) Size Surface Type and Composition Atmosphere Temperature Name Pluto 1 tiny 2350 km solid rock, methane ice none (methane) very cold -230 C 2 medium large 49,500 km liquid hydrogen, helium thick hydrogen, helium, methane very cold C -220 Neptune 3 medium large 51,100 km liquid hydrogen, helium thick hydrogen, helium, methane very cold C -210 Uranus 4 large 120,500 km liquid hydrogen cold -180 C Saturn 5 very large 143,000 km liquid hydrogen thick hydrogen, helium thick hydrogen, helium cold -150 C Jupiter 6 small km solid rock, water ice thin carbon dioxide moderate -23 C Mars 7 medium small 12,756 km solid rock, liquid water, carbon compounds medium nitrogen, oxygen moderate 21 C Earth 8 medium small 12,100 km solid rock thick carbon dioxide very hot 480 C Venus 9 tiny 4878 km solid rock none (helium) EL8_2014 6786 variable range 180 to 400 C Mercury 153 Student PLANET CHARACTERISTICS Size Surface Type and Composition Atmosphere Temperature Name 1 2 3 4 5 6 7 8 9 EL8_2014 154 PLANETARY EXPLORATION & EXTREME LIFE FORMS (Differentiated Lab) Revised by: University of Miami – Science Made Sensible Fellows Next Generation Sunshine State Standards: SC.8.E.5.1 Recognize that there are enormous distances between objects in space and apply our knowledge of light and space travel to understand this distance. SC.8.E.5.3 Distinguish the hierarchical relationships between planets and other astronomical bodies relative to solar system, galaxy, and universe, including distance, size, and composition. SC.8.E.5.7 Compare and contrast the properties of objects in the solar system including the Sun, planets, and moons to those of Earth, such as gravitational force, distance from the Sun, speed, movement, temperature, and atmospheric conditions. SC.7.L.15.3 Explore the scientific theory of evolution by relating how the inability of a species to adapt within a changing environment may contribute to the extinction of that species. SC.7.L.16.2 Determine the probabilities for genotype and phenotype combinations using Punnett Squares and pedigrees. SC.7.L.17.3 Describe and investigate various limiting factors in the local ecosystem and their impact on native populations, including food, shelter, water, space, disease, parasitism, predation, and nesting sites. Objective: Students will research a planet in our solar system, including information about the atmosphere, surface conditions, etc. Then they will have to design an alien life form that would be adapted to live on their planet. They will present their planet research and alien life forms to the class. They will also use a Punnett Square to predict offspring genotype and phenotype. Engage: Introduce adaption and extreme environments. Scientists are finding organisms on Earth that live in extreme conditions previously thought not able to support life. Some of these extreme environments include the harsh, dry, cold valleys of Antarctica and the bottom of the ocean under high pressure, no oxygen and no light. If life forms on other planets were to exist, what conditions would they face? How would they survive? What type of adaptations might they need? Explain that students will first research a planet, and then create a life form that had adapted to survive the conditions on their planet. Materials: computers with internet access books on the planets construction paper markers/crayons/colored pencils Teacher Notes: Assign one group to each planet excluding Earth. For the first part of the activity, students will research their planet, filling in a data sheet. All information can be found on the websites provided on the student handouts. Emphasize the importance of using appropriate internet sources, no Wikipedia. Once students have completed their planet worksheet, they should start on the alien life form worksheet. They will also draw their life form on construction paper. Groups will present both their planet research and their aliens including explanations of its specific adaptations that allow it to survive on their planet. Upper level students could be required to do a PowerPoint presentation. EL8_2014 155 Student Name: ____________________________________ Date: ___________________ Pd: __________ Planet Research Worksheet Fill in the worksheet below about your assigned planet; be sure to include units where necessary. Some helpful websites for your research are: http://nineplanets.org/ http://solarsystem.nasa.gov/index.cfm www.windows2universe.org/our_solar_system/solar_system.html www.exploratorium.edu/ronh/weight/index.html Planet: _____________________________ Planetary Symbol: ___________________________ Diameter: ___________________________ Mass: _____________________________________ Order from the Sun: ___________________ Distance from the Sun: _______________________ Gravity: ____________________________ Gravity compared to Earth: ___________________ If you weigh 100 lbs. on Earth, how much would you weigh on your planet? _________________ Temperature Range: ___________________ Average Temperature ________________________ Length of Day (rotation period): _________ Length of year (revolution period): ______________ Tilt of axis: __________ Eccentricity of Orbit: ________ Number of Satellites: ________ What is the atmosphere like on your planet? What gases? Poisonous? Dry? Etc. Describe the surface of your planet. EL8_2014 156 Describe what your planet looks like including any unique features such as rings. In complete sentences, list 5 additional interesting facts about your planet that are not already discussed on this worksheet. EL8_2014 157 Student Name: ____________________________________ Date: ___________________ Pd: __________ Extreme Alien Life Forms You will create an alien life form that has adaptations enabling it to survive on your assigned planet. Keep in mind the information you learned about your planet during your research. Complete the questions below, and then draw your life form on the provided construction paper. Make sure to label the aspects of your life form that let it survive on your planet. Include your life form’s name, your planet, and your name and class period on the front of your drawing. You will be presenting your drawings to the class. Your Planet: The name of your life form: Describe the habitat and climate in which your life form lives: How does it move? Include both the form and method of locomotion. (For example: The miniature Mars Gopher leaps on powerful hind legs.) What does it eat or use as nutrients? Is it herbivorous, carnivorous, omnivorous, or other? What is its main food and how does it acquire this food? What other creatures does it prey on, if any? How does it defend itself against predators? EL8_2014 158 Describe other adaptations your life form has developed to cope with your planet’s unique environment. Suppose two alien creatures mated. One was tall and the other was short. Using a Punnett Square, predict the offspring’s possible heredity of the tall gene. Each parent has two alleles for the height gene. Dad is homozygous tall (TT) and mom is homozygous short (tt). Predict the genotype (genetic make-up) and phenotype (physical characteristics) for the offspring. Dad → ______ ______ ______ Offspring ______ Mom ↑ The resulting offspring: Genotype: __________% TT Phenotype: __________% tall Genotype TT (homozygous tall) tt (homozygous short) Tt (heterozygous) EL8_2014 __________% tt __________% Tt __________% short Phenotype Tall Short Tall 159 STUDENT HANDOUT DIFFERENTIATED INSTRUCTION: GUIDED/OPEN INQUIRY PLANETARY EXPLORATION AND EXTREME ALIEN LIFE FORMS Objectives/Purpose: In this investigation students will: Compare and contrast the properties of objects in the solar system (gravitational force, distance from the Sun, speed, movement, temperature, and atmospheric conditions.) Describe and investigate various limiting factors in the local ecosystem and their impact on native populations, including food, shelter, water, space for these “alien” environments Relate how the inability of an “alien” species to adapt within a changing environment may contribute to the extinction of that species Demonstrate Achievement of the following Goals: Develop a problem statement based on the concept of “planetary exploration and extreme alien life forms” that you would like to investigate. Model the concept/idea that you outlined in your problem statement above. How does the model demonstrate the concept that you investigated? Remember to use the “Claim, Evidence & Reasoning” rubric to defend your claims. EL8_2014 160 ANTI-DISCRIMINATION POLICY Federal and State Laws The School Board of Miami-Dade County, Florida adheres to a policy of nondiscrimination in employment and educational programs/activities and strives affirmatively to provide equal opportunity for all as required by law: Title VI of the Civil Rights Act of 1964 - prohibits discrimination on the basis of race, color, religion, or national origin. Title VII of the Civil Rights Act of 1964, as amended - prohibits discrimination in employment on the basis of race, color, religion, gender, or national origin. Title IX of the Educational Amendments of 1972 - prohibits discrimination on the basis of gender. Age Discrimination in Employment Act of 1967 (ADEA), as amended - prohibits discrimination on the basis of age with respect to individuals who are at least 40. The Equal Pay Act of 1963, as amended - prohibits gender discrimination in payment of wages to women and men performing substantially equal work in the same establishment. Section 504 of the Rehabilitation Act of 1973 - prohibits discrimination against the disabled. Americans with Disabilities Act of 1990 (ADA) - prohibits discrimination against individuals with disabilities in employment, public service, public accommodations and telecommunications. The Family and Medical Leave Act of 1993 (FMLA) - requires covered employers to provide up to 12 weeks of unpaid, job-protected leave to “eligible” employees for certain family and medical reasons. The Pregnancy Discrimination Act of 1978 - prohibits discrimination in employment on the basis of pregnancy, childbirth, or related medical conditions. Florida Educational Equity Act (FEEA) - prohibits discrimination on the basis of race, gender, national origin, marital status, or handicap against a student or employee. Florida Civil Rights Act of 1992 - secures for all individuals within the state freedom from discrimination because of race, color, religion, sex, national origin, age, handicap, or marital status. Veterans are provided re-employment rights in accordance with P.L. 93-508 (Federal Law) and Section 295.07 (Florida Statutes), which stipulates categorical preferences for employment. Revised 9/2008 EL8_2014 161