Assignment 1: Math Foundations of Quantum Mechanics
advertisement
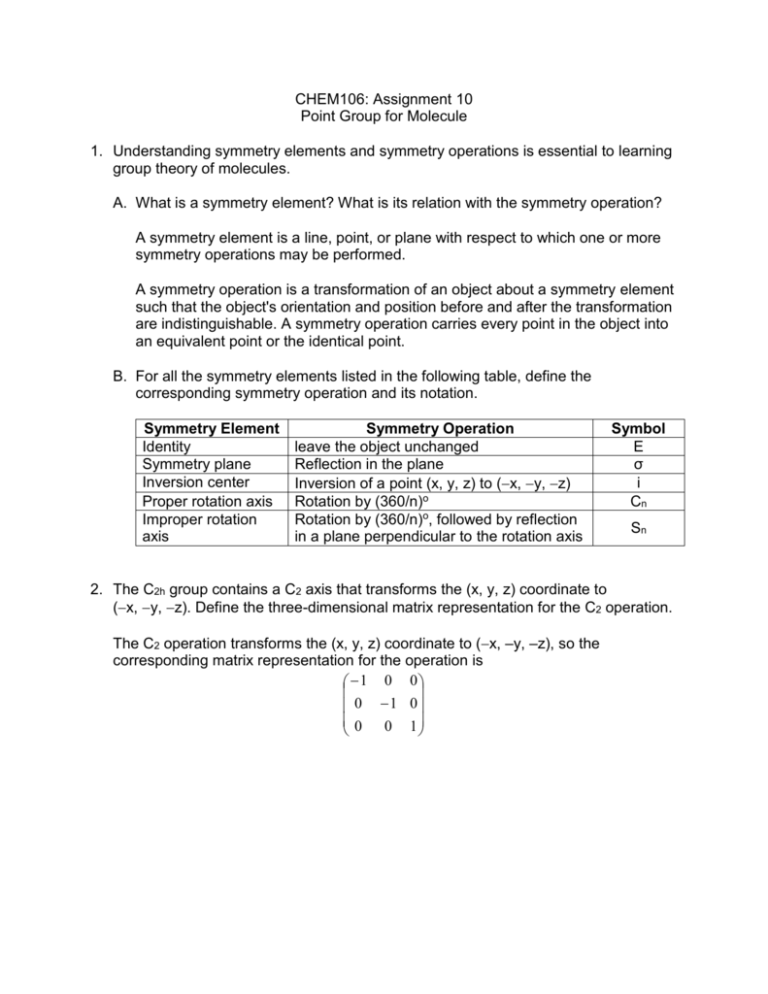
CHEM106: Assignment 10 Point Group for Molecule 1. Understanding symmetry elements and symmetry operations is essential to learning group theory of molecules. A. What is a symmetry element? What is its relation with the symmetry operation? A symmetry element is a line, point, or plane with respect to which one or more symmetry operations may be performed. A symmetry operation is a transformation of an object about a symmetry element such that the object's orientation and position before and after the transformation are indistinguishable. A symmetry operation carries every point in the object into an equivalent point or the identical point. B. For all the symmetry elements listed in the following table, define the corresponding symmetry operation and its notation. Symmetry Element Identity Symmetry plane Inversion center Proper rotation axis Improper rotation axis Symmetry Operation leave the object unchanged Reflection in the plane Inversion of a point (x, y, z) to (x, y, z) Rotation by (360/n)o Rotation by (360/n)o, followed by reflection in a plane perpendicular to the rotation axis Symbol E σ i Cn Sn 2. The C2h group contains a C2 axis that transforms the (x, y, z) coordinate to (x, y, z). Define the three-dimensional matrix representation for the C2 operation. The C2 operation transforms the (x, y, z) coordinate to (x, –y, –z), so the corresponding matrix representation for the operation is 1 0 0 0 1 0 0 0 1 3. Identify all the symmetry elements in the chloroform (CHCl3) molecule, and assign its point group. As shown in the figure below, there is a c3 axis of rotation that passes through the C-H bond. It is also the principal axis of rotation. There are three σv mirror planes (each defined by the H-C-Cl bond plane). Symmetry elements: E, C3, v, v', v'' Point Group: C3v 4. Determine the symmetry group for the following molecules. A. NH3 C3V B. CCl4 Td C. Planar BF3 D2h D. H2C=CBr2 C2V E. C6H6 D6h