Supplementary Table 1
advertisement

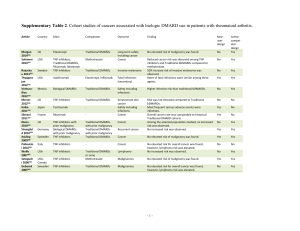
Supplementary Table 1. Cohort studies of infections associated with biologic DMARD use in patients with rheumatoid arthritis. Article Country Main Comparator Outcome Finding Newuser design Chiang 2014S1 Bountha vong 2014S2 Alawneh 2014S3 Yun 2014S4 Yoo 2014S5 Curtis 2014S6 Cho 2014S7 Taiwan Etanercept Adalimumab Serious infection Adjusted HR was 2.04 for etanercept. Yes Activecompar ator design Yes USA Etanercept Adalimumab, Infliximab Infection event (secondary) No significant difference in rates of infections Yes Yes Jordan Infliximab No Yes TNF inhibitors Yes Infliximab Adalimumab Adjusted HR 0.80 for abatacept and 0.83 for etanercept. No significant difference in rates of infections No Korea Yes Yes USA TNF inhibitors Rituximab, Abatacept HR 2.3 for infliximab compared to etanercept Yes Yes Korea, Japan Korean TNF inhibitor users Japanese TNF inhibitor users Higher unadjusted incidence rate of infection in Japan Yes Yes Ke 2013S8 Koike 2014S9 Lee 2013S10 Johnston 2013S11 Taiwan TNF inhibitors Traditional DMARDs Severe infection and tuberculosis Hospitalized infection Serious infection and tuberculosis Hospitalized bacterial infections Serious adverse events including infection Active tuberculosis No significant difference among groups USA Adalimumab, Etanercept Abatacept, Rituximab Adjusted HR was 4.87 for TNF inhibitor users. Yes Yes Japan Tocilizumab - Serious infection Yes No Korea TNF inhibitors General population No No USA Rituximab as second agent Compared to rituximab, adjusted HR were elevated for adalimumab, etanercept, infliximab. Yes Yes Bello 2012S12 van Dartel 2013S13 Curtis 2012S14 Atzeni 2012S15 Thyagara jan 2012S16 Dewedar 2012S17 VenturaRíos 2012S18 Italy Abatacept, Adalimumab, Etanercept, Infliximab as second agents Biological DMARDs Mycobacterial infections Infections Higher age, longer RA duration, comorbidity, and glucocorticoids were risk factors. Standardize incidence ratio of 6.4. General population Influenza-like illness Increased incidence of influenza-like illness No No Netherla nds Etanercept Adalimumab, Infliximab Serious infections Adjusted HR was significantly lower for etanercept. Yes Yes USA Infliximab Serious infections Infliximab had a significantly higher adjusted rates. Yes Yes Italy Infliximab Serious infections Yes Adalimumab Adjusted HR was increased for infliximab and adalimumab compared to etanercept. Rates of fatal infections were similar among three agents. Yes USA Etanercept, Adalimumab Adalimumab, Etanercept Etanercept, Infliximab Yes Yes Saudi Arabia Mexico TNF inhibitors Traditional DMARDs Unadjusted rates were not significantly different. No Yes Biological DMARDs Traditional DMARDs Adverse drug effects including infections Safety including infections Higher infection risk than traditional bDMARDs. No Yes Fatal infections (secondary) -1- NguyenKhoa 2012S19 Gallowa y 2013S20 Sakai 2012S21 Grijalva 2011S22 Lang 2012S23 USA TNF inhibitor switcher TNF inhibitor non-switcher First significant infection Adjusted HR was 0.93 No Yes UK Biological DMARDs Traditional DMARDs Soft tissue infections Adjusted HR for zoster was high for bDMARDs. No Yes Japan TNF inhibitors Traditional DMARDs Serious infections Yes Yes USA TNF inhibitors Traditional DMARDs Yes Yes Germany Tocilizumab - Hospitalized infections Infections Yes No Koike 2011S24 Strangfel d 2011S25 Gallowa y 2011S26 Curtis 2011S27 Komano 2011S28 Lane 2011S29 Momoha ra 2011S30 Gallowa y 2011S31 Gottenb erg 2010S32 Suwanna lai 2009S33 Peña-Sag redo 2009S34 Dixon 2010S35 Greenbe rg 2010S36 Aggarwa l 2009S37 Strangfel d 2009S38 Peña-Sag redo 2008S39 Japan Tocilizumab - Yes No Germany TNF inhibitors Traditional DMARDs Safety including infections Serious infections Yes Yes UK TNF inhibitors Traditional DMARDs Septic arthritis Adjusted incidence rate ratio was elevated during the first year for biological bDMARDs. TNF inhibitor initiation was not associated with an increased risk of hospitalized serious infections. Among tocilizumab users, risk factors included longer disease duration and exposure to multiple previous treatments. Most frequent serious adverse events were infections. Adjusted incidence rate ratio was 1.8 for TNF inhibitors. Adjusted HR was 2.3 for TNF inhibitors. No Yes USA Biological DMARDs Serious infections Patient risk factors contributed more to infections. Yes Yes Japan TNF inhibitors Biological DMARD switchers Traditional DMARDs Serious infections Adjusted RR was 2.37 for TNF inhibitors. No Yes USA TNF inhibitors Traditional DMARDs Adjusted HR was 1.24 for TNF inhibitors. Yes Yes Japan Biological DMARDs Traditional DMARDs Hospitalized infections Acute surgical-site infections Adjusted RR was elevated for biological bDMARDs No Yes UK TNF inhibitors Traditional DMARDs Serious infections No Yes France Rituximab - Severe infections Adjusted HR was 1.8 for TNF inhibitors during the first 6 months. Baseline comorbidities were associated with infections. Yes No Thailand Etanercept Infliximab Serious infections Overall infection rate was higher among infliximab users. Yes Yes Spain TNF inhibitors Traditional DMARDs Non-typhi Salmonella infections Infection incidence was not elevated, but was more severe. No Yes UK Etanercept Tuberculosis Yes Other Traditional DMARDs Infections including opportunistic No Yes - Tuberculosis Tuberculosis incidence were elevated in infliximab and adalimumab compared to etanercept. TNF inhibitors and methotrexate users had increased risk of infection compared to other Traditional bDMARDs No active tuberculosis was found. Yes USA Infliximab, adalimumab TNF inhibitors with or without methotrexate, methotrexate Etanercept Yes No Germany TNF inhibitors Traditional DMARDs Herpes zoster Yes Yes Spain TNF inhibitors - Listeria infections No increased risk for TNF inhibitor class, HR 1.82 for infliximab and adalimumab. Incidence was higher than general population. Yes No USA -2- Favalli 2009S40 Garcia-Vi dal 2009S41 Curtis 2007S42 Dixon 2007S43 Curtis 2007S44 Seong 2007S45 den Broeder 2007S46 Salliot 2007S47 Dixon 2006S48 Giles 2006S49 Italy Infliximab Serious infections Incidence did not differ among TNF inhibitors. Yes No Infliximab Adalimumab, Etanercept - Spain Opportunistic infection Incidence was elevated during first year of treatment. Yes No USA TNF inhibitors Methotrexate Bacterial infections Yes Yes UK TNF inhibitors Traditional DMARDs Serious infection No Yes USA TNF inhibitors Methotrexate No Yes Korea TNF inhibitors General population Serious bacterial infection Tuberculosis Incidence of infection was elevated during first 6 months of TNF inhibitor treatment. Incidence rate ratio 4.6 for TNF inhibitors in first 90 days. Adjusted HR was 1.9 for TNF inhibitors. No No Netherla nds TNF inhibitors Traditional DMARDs Surgical site infections Adjusted RR was 8.9 for TNF inhibitor users compared to general population. No elevated risk of surgical site infection was associated with TNF inhibitors. No Yes France TNF inhibitors Traditional DMARDs Serious infections No Yes UK TNF inhibitors Traditional DMARDs Serious infections No Yes USA TNF inhibitors Traditional DMARDs No Yes Listing 2005S50 Germany Etanercept, Infliximab Traditional DMARDs Adjusted RR was non-significant. Yes Yes Maillard 2005S51 Neven 2005S52 France Infliximab - No Infliximab - Older age and high-dose glucocorticoid therapy were associated with an increased risk. Infection was the most common adverse event. No Netherla nds Serious postoperative orthopedic infections Serious and nonserious infections Severe pyogenic infections Adverse drug effects including infections Relative risk was 3.1 compared to period before TNF inhibitor initiation. Serious skin and soft tissue infection was increased in TNF inhibitors. Significant association between TNF inhibitors and postoperative infection. Yes No References: S1. Chiang, Y.-C., Kuo, L.-N., Yen, Y.-H., Tang, C.-H. & Chen, H.-Y. Infection risk in patients with rheumatoid arthritis treated with etanercept or adalimumab. Comput Methods Programs Biomed 116, 319–327 (2014). S2. Bounthavong, M., Madkour, N. & Kazerooni, R. Retrospective cohort study of anti-tumor necrosis factor agent use in a veteran population. PeerJ 2, (2014). S3. Alawneh, K. M. et al. Anti-TNF therapy in Jordan: a focus on severe infections and tuberculosis. Biologics 8, 193–198 (2014). S4. Yun, H. et al. Risk of hospitalised infection in rheumatoid arthritis patients receiving biologics following a previous infection while on treatment with anti-TNF therapy. Ann Rheum Dis (2014). doi:10.1136/annrheumdis-2013-204011 S5. Yoo, I. K. et al. Incidences of serious infections and tuberculosis among patients receiving anti-tumor necrosis factor-alpha therapy. Yonsei Med J 55, 442– 448 (2014). S6. Curtis, J. R. et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 66, 990–997 (2014). S7. Cho, S.-K. et al. A comparison of incidence and risk factors for serious adverse events in rheumatoid arthritis patients with etanercept or adalimumab in Korea and Japan. Mod Rheumatol 24, 572–579 (2014). S8. Ke, W.-M., Chen, L.-S., Parng, I.-M., Chen, W.-W. & On, A. W. F. Risk of tuberculosis in rheumatoid arthritis patients on tumour necrosis factor-alpha -3- inhibitor treatment in Taiwan. Int J Tuberc Lung Dis 17, 1590–1595 (2013). S9. Koike, T. et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 41, (2014). S10.Lee, S. K. et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung 191, 565–571 (2013). S11.Johnston, S. S. et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Seminars in Arthritis and Rheumatism 43, 39–47 (2013). S12.Bello, S. L. et al. Incidence of influenza-like illness into a cohort of patients affected by chronic inflammatory rheumatism and treated with biological agents. Reumatismo 64, (2012). S13.Van Dartel, S. A. A. et al. Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis 72, (2013). S14.Curtis, J. R. et al. Use of a disease risk score to compare serious infections associated with anti-tumor necrosis factor therapy among high- versus lower-risk rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 64, 1480–1489 (2012). S15.Atzeni, F. et al. Long-term anti-TNF therapy and the risk of serious infections in a cohort of patients with rheumatoid arthritis: comparison of adalimumab, etanercept and infliximab in the GISEA registry. Autoimmun Rev 12, 225–229 (2012). S16.Thyagarajan, V., Norman, H., Alexander, K. A., Napalkov, P. & Enger, C. Risk of mortality, fatal infection, and fatal malignancy related to use of anti-tumor necrosis factor-alpha biologics by rheumatoid arthritis patients. Semin Arthritis Rheum 42, 223–233 (2012). S17.Dewedar, A. M. et al. Lack of adverse effect of anti-tumor necrosis factor-alpha biologics in treatment of rheumatoid arthritis: 5 years follow-up. Int J Rheum Dis 15, 330–335 (2012). S18.Ventura-Rios, L. et al. Patient survival and safety with biologic therapy. Results of the Mexican National Registry Biobadamex 1.0. Reumatol Clin 8, 189– 194 (2012). S19.Nguyen-Khoa, B.-A. et al. Risk of significant infection in rheumatoid arthritis patients switching anti-tumor necrosis factor-alpha drugs. Semin Arthritis Rheum 42, 119–126 (2012). S20.Galloway, J. B. et al. Risk of skin and soft tissue infections (including shingles) in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 72, 229–234 (2013). S21.Sakai, R. et al. Time-dependent increased risk for serious infection from continuous use of tumor necrosis factor antagonists over three years in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 64, 1125–1134 (2012). S22.Grijalva, C. G. et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 306, 2331–2339 (2011). S23.Lang, V. R. et al. Risk of infections in rheumatoid arthritis patients treated with tocilizumab. Rheumatology (Oxford) 51, 852–857 (2012). S24.Koike, T. et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 70, 2148– 2151 (2011). S25.Strangfeld, A. et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann. Rheum. Dis. 70, 1914–1920 (2011). S26.Galloway, J. B. et al. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 70, 1810–1814 (2011). S27.Curtis, J. R. et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis 70, 1401–1406 (2011). S28.Komano, Y. et al. Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the Registry of Japanese Rheumatoid Arthritis Patients for Longterm Safety. J Rheumatol 38, 1258–1264 (2011). S29.Lane, M. A. et al. TNF-alpha antagonist use and risk of hospitalization for infection in a national cohort of veterans with rheumatoid arthritis. Medicine (Baltimore) 90, 139–145 (2011). -4- S30.Momohara, S. et al. Prosthetic joint infection after total hip or knee arthroplasty in rheumatoid arthritis patients treated with nonbiologic and biologic disease-modifying antirheumatic drugs. Mod Rheumatol 21, 469–475 (2011). S31.Galloway, J. B. et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50, 124–131 (2011). S32.Gottenberg, J.-E. et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum 62, 2625–2632 (2010). S33.Suwannalai, P., Auethavekiat, P., Udomsubpayakul, U. & Janvitayanujit, S. The infectious profiles of anti-tumor necrosis factor agents in a Thai population: a retrospective study a the university-based hospital. Int J Rheum Dis 12, 118–124 (2009). S34.Pena-Sagredo, J. L. et al. Non-typhi Salmonella infection in patients with rheumatic diseases on TNF-alpha antagonist therapy. Clin Exp Rheumatol 27, 920–925 (2009). S35.Dixon, W. G. et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 69, 522–528 (2010). S36.Greenberg, J. D. et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis 69, 380–386 (2010). S37.Aggarwal, R., Manadan, A. M., Poliyedath, A., Sequeira, W. & Block, J. A. Safety of etanercept in patients at high risk for mycobacterial tuberculosis infections. J Rheumatol 36, 914–917 (2009). S38.Strangfeld, A. et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA 301, 737–744 (2009). S39.Pena-Sagredo, J. L. et al. Listeria monocytogenes infection in patients with rheumatic diseases on TNF-alpha antagonist therapy: the Spanish Study Group experience. Clin Exp Rheumatol 26, 854–859 (2008). S40.Favalli, E. G. et al. Serious infections during anti-TNFalpha treatment in rheumatoid arthritis patients. Autoimmun Rev 8, 266–273 (2009). S41.Garcia-Vidal, C. et al. Risk factors for opportunistic infections in infliximab-treated patients: the importance of screening in prevention. Eur J Clin Microbiol Infect Dis 28, 331–337 (2009). S42.Curtis, J. R. et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum 56, 1125–1133 (2007). S43.Dixon, W. G. et al. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum 56, 2896–2904 (2007). S44.Curtis, J. R. et al. Drug-specific and time-dependent risks of bacterial infection among patients with rheumatoid arthritis who were exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum 56, 4226–4227 (2007). S45.Seong, S.-S. et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol 34, 706–711 (2007). S46.Den Broeder, A. A. et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol 34, 689–695 (2007). S47.Salliot, C. et al. Infections during tumour necrosis factor-alpha blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology (Oxford) 46, 327–334 (2007). S48.Dixon, W. G. et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 54, 2368–2376 (2006). S49.Giles, J. T. et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum 55, 333–337 (2006). S50.Listing, J. et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 52, 3403–3412 (2005). S51.Maillard, H. et al. Severe pyogenic infections in patients taking infliximab: a regional cohort study. Joint Bone Spine 72, 330–334 (2005). -5- S52.Neven, N. et al. Adverse events in patients with rheumatoid arthritis treated with infliximab in daily clinical practice. Ann Rheum Dis 64, 645–646 (2005). -6-