Chapters
advertisement
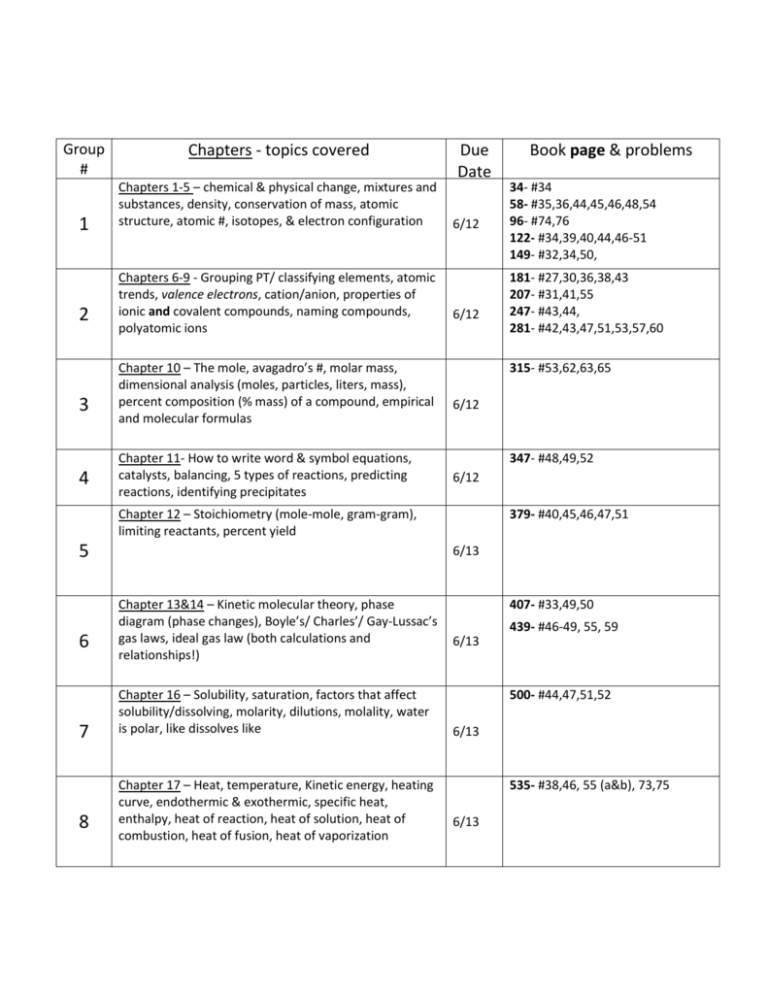
Group # 1 2 3 4 Chapters - topics covered Chapters 1-5 – chemical & physical change, mixtures and substances, density, conservation of mass, atomic structure, atomic #, isotopes, & electron configuration Chapters 6-9 - Grouping PT/ classifying elements, atomic trends, valence electrons, cation/anion, properties of ionic and covalent compounds, naming compounds, polyatomic ions Chapter 10 – The mole, avagadro’s #, molar mass, dimensional analysis (moles, particles, liters, mass), percent composition (% mass) of a compound, empirical and molecular formulas Chapter 11- How to write word & symbol equations, catalysts, balancing, 5 types of reactions, predicting reactions, identifying precipitates Due Date 6/12 6/12 6 7 8 34- #34 58- #35,36,44,45,46,48,54 96- #74,76 122- #34,39,40,44,46-51 149- #32,34,50, 181- #27,30,36,38,43 207- #31,41,55 247- #43,44, 281- #42,43,47,51,53,57,60 315- #53,62,63,65 6/12 347- #48,49,52 6/12 Chapter 12 – Stoichiometry (mole-mole, gram-gram), limiting reactants, percent yield 5 Book page & problems 379- #40,45,46,47,51 6/13 Chapter 13&14 – Kinetic molecular theory, phase diagram (phase changes), Boyle’s/ Charles’/ Gay-Lussac’s gas laws, ideal gas law (both calculations and relationships!) Chapter 16 – Solubility, saturation, factors that affect solubility/dissolving, molarity, dilutions, molality, water is polar, like dissolves like Chapter 17 – Heat, temperature, Kinetic energy, heating curve, endothermic & exothermic, specific heat, enthalpy, heat of reaction, heat of solution, heat of combustion, heat of fusion, heat of vaporization 407- #33,49,50 439- #46-49, 55, 59 6/13 500- #44,47,51,52 6/13 535- #38,46, 55 (a&b), 73,75 6/13 Acids & Bases (chapter 19): General Neutralization reactions (acid + base -> ?) Calculating pH & pOH Concentration of H+ ions vs pH o Would a low pH have a high concentration of H+ ions or low concentration of H+ ions? (hint: low pH means acidic) o Would a high pH have a high concentration of H+ ions or low concentration of H+ ions? Concentration of OH- ions vs pH o Would a low pH have a high concentration of OH- ions or low concentration of H+ ions o Would a low pH have a high concentration of OH- ions or low concentration of H+ ions Loose Ends: Polarity: This is more like a scale (like the pH scale) where you can be nonpolar, slightly polar, kind of polar, and REALLY polar. The like-dissolves-like principle still applies: slightly polar dissolves slightly polar really well and really polar dissolves really polar. This slightly-kinda polar is sometimes called “polar covalent”. (Water is polar covalent, which is STILL polar) Covalent bonds: We didn’t spend a lot of time on these. As far as properties are concerned, they are (generally) the opposite of ionic. If ionic is strong, covalent is weak. If ionic has high melting/boiling point, covalent has a low melting/boiling point. Ionic can conduct electricity (electrolyte), covalent compounds generally cannot.








