NTReport
advertisement

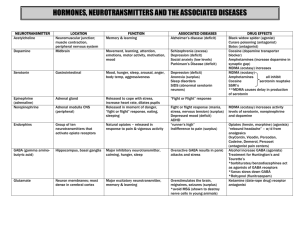
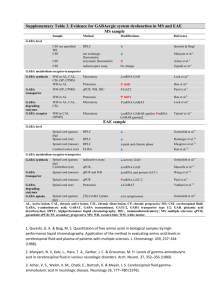
Surface Plasmon Resonance Feasibility Experiments Neurotransmitter Interaction with Sparsely Tethered Lipid Membranes (stBLM) Brian Josey, Stefanie Rintoul, Siddharth Shenoy, Frank Heinrich, Mathias Lösche, Robert Cantor November 2013 Summary 2 Samples 2 Results 4 GABA Control 4 GABA 6 GABA Neutron Reflectometry 11 Acetylcholine Chloride Control 13 Acetylcholine Chloride 16 Serotonin Results 22 Serotonin Neutron Reflectometry 24 Discussion 26 Comparison to Wang, C. et al. 26 Further Experiments 27 Discussion Notes 28 Notes from a discussion with Robert Cantor – Summer 2013 28 Notes from a discussion with Vitalii Silin – Summer 2013 28 Notes from a discussion with Robert Cantor – Summer 2013 28 Summary SPR measurements studying the bilayer association of GABA, acetylcholine and serotonin with 70:30 POPC:POPG tethered lipid membranes were performed. All three of the neurotransmitters were found to bind to the lipid bilayer. In a single-binding process Langmuir isotherm model, GABA binds with a K D of about 0.9±0.6 µM, acetylcholine exhibits a KD of about 10 mM and serotonin exhibits a KD of 0.08±0.2 µM. Estimates for maximum surface densities for GABA vary between one GABA molecule per lipid and one GABA molecule per 5 lipid molecules. Association of acetylcholine and serotonin are linked to observable bilayer thinning. Therefore surface coverage estimates cannot be obtained. Samples Tethered lipid bilayer membranes (tBLMs) completed with a 70:30 mixture of POPC and POPG were prepared on gold-coated surfaced coated with a 70:30 β-mercaptoethanol : HC18 SAMs. tBLMs were exposed to a series of concentrations of either GABA , acetylcholine or serotonin and the SPR signal was recorded for every concentration without rinsing with pure buffer in-between. All experiments were conducted using an instrument from SPR Biosystems (Germantown, MD). GABA (A5835-10G), Acetylcholine Chloride (A6625-25G) and Serotonin hydrochloride (H9523-100MG) were purchased from Sigma-Aldrich. Table 1. List of Samples. Sample Date Buffer stBLM Neurotransmitter stBLM preparation 1 12/12/2011 100 mM NaCl 10 mM HEPES pH 7.5 70:30 POPC:POPG GABA 0.01µM-600mM rapid solvent exchange at 40ºC 2 12/13/2011 100 mM NaCl 10 mM HEPES pH 7.5 70:30 POPC:POPG GABA 0.01µM-500mM rapid solvent exchange at 25ºC 3 12/14/2011 100 mM NaCl 10 mM HEPES pH 7.5 N/A SAM only GABA 0.01µM-400mM N/A 4 5/23/2012 100 mM NaCl 10 mM NaH2PO4 pH 7.5 70:30 POPC:POPG Acetylcholine Chloride .01µM–5.5 mM rapid solvent exchange at 40ºC 5 6/8/2012 100 mM NaCl 10 mM NaH2PO4 pH 7.5 70:30 POPC:POPG Acetylcholine Chloride 0.01µM-400mM N/A 6 6/11/2012 100 mM NaCl 10 mM NaH2PO4 pH 7.5 N/A SAM only Acetylcholine Chloride 0.01µM-400mM rapid solvent exchange at 40ºC 7 6/28/2012 100 mM NaCl 10 mM NaH2PO4 pH 7.5 70:30 POPC:POPG GABA 0.01 µM - 250 µM rapid solvent exchange at 40ºC 8 8/3/2012 100 mM NaCl 10 mM NaH2PO4 pH 7.5 70:30 POPC:POPG Acetylcholine Chloride 0.01µM-400mM rapid solvent exchange at 40ºC 9 2/28/2013 100 mM NaCl 10 mM HEPES pH 7.5 70:30 POPC:POPG GABA 0.01 µM – 500 mM Rapid solvent exchange at 22ºC 10 6/7/2013 100 mM NaCl 10 mM HEPES pH 7.5 70:30 POPC:POPG Serotonin 0.01 µM – 10 mM Rapid solvent exchange at 22ºC Figure 1: GABA Figure 2: Acetylcholine chloride Figure 3: Serotonin hydrochloride Results GABA Control A gold surface coated with a SAM from a 70:30 ethanolic solution of βmercaptoethanol and HC18 tether molecules was exposed to GABA concentrations between 10nM and 400mM (see Figure 4). There is no observable change of the SPR signal when adding up to 1mM of GABA. For concentrations higher than 1mM an increase of the SPR signal that is strictly linear with GABA concentration is observed. This linear increase does not show saturation and can be attributed exclusively to a change of the reflective index of the bulk solvent rather than to surface-bound GABA. This linear relation between the change of the bulk refractive index and the SPR signal will be used to distinguish between surface-bound GABA and change of the bulk solvent refractive index at high GABA concentrations in the following experiments using lipid bilayers. To achieve this, a linear function will be fitted to the high-concentration region of the SPR data and subtracted. In case that the high-concentration region contains signal that arises from surface-bound GABA, the corrected signal will remain above background. In case that the dissociation constant KD lies within the high-concentration region, a correction of the SPR signal to a constant value is not possible, and therefore, this situation can be identified. Figure 4: Sample 3, no lipid bilayer - SAM only, GABA. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. There is no binding to the SAM in the low concentration region up to 1 mM GABA. Above 1 mM GABA a continuous change of the refractive index of the bulk solution due to the high concentration of GABA is observed. GABA Three GABA experiments were performed by exposing a tBLM composed of 70:30 POPC:POPG to GABA concentrations between 10nM and 600mM (see Figure 5), between 10nM and 500nM (see Figure 6), and between 10nM and 250µM (see Figure 7). A summary of all three obtained dissociation constants K D and maximum SPR signal values (pixel changes) can be found in Table . An average KD value of 0.92±0.54 µM was observed and an average saturation of 1.82±1.68 pixel change was fitted to the binding curves. While those values show a large variation, all measured binding curves can be fit with a single sigmoidal binding curve and no additional or increased binding can be detected up to 100 mM GABA. Above this concentration the correction of the SPR data for the change of the bulk refractive index does not yield a constant signal anymore. This is either due to a failure of the correction or due to binding of GABA to the interface above 100 mM. The average pixel change at saturation of 1.82±1.68 corresponds to an approximated 1 GABA molecule per 2.5 lipid molecules assuming an area per lipid of 65 Å2. Sample kd (µM) bmax 1 1.5±0.6 3.7±0.2 2 0.9±4.9 1.2±0.7 7 0.4±0.3 0.6±0.2 average ± sd 0.9±0.6 1.8±1.7 surface coverage estimate (1 Pixel = 5.8 ng/cm2, area per lipid 65Å2) 10.6 ng/cm2 1 neurotransmitter per 2.5 lipid molecules Table 2: Experimentally derived dissociation constants and SPR saturation values for experiments using GABA. A fourth SPR experiment was performed independently of the others using neurotransmitter concentrations from 10nM to 10 mM and a buffer of 100mM NaCl and 10 mM HEPES. A negative change in the SPR pixel value was observed. Fitting this data to a Langmuir isotherm with negative saturation gave KD value of 1.28±1.01 µM was observed and an average saturation of 1.28±0.19 pixel change (see Figure 8). Figure 4: Sample 1, 70:30 POPC:POPG, GABA, bilayer preparation at 40ºC. Top: SPR raw data, pixel change vs. time. Pixel change is related to the change of refractive index at the interface, and, therefore the associated mass. Different colors indicate different GABA concentration sequentially added to the stBLM. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. The red data points are directly obtained from the top panel, the blue data points are the red data corrected for the refractive index change of the bulk solvent. An equilibrium dissociation constant of 1.4566±0.568 µM was determined and a saturation pixel change of 3.7292±0.141 pixels. Figure 6: Sample 2, 70:30 POPC:POPG, GABA, bilayer preparation at room temperature. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. The red data points are directly obtained from the top panel, the blue data points are the red data corrected for the change of the refractive index of the bulk solvent. An equilibrium dissociation constant of 0.93666±4.93 µM was determined and a saturation pixel change of 1.2±0.708 pixels. Figure 7: Sample 7, 70:30 POPC:POPG, GABA, bilayer preparation at 40ºC. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. An equilibrium dissociation constant of 0.36731±0.301 µM was determined and a saturation pixel change of 0.55664±0.12 pixels. Figure 5: Sample 9, 70:30 POPC:POPG, GABA, bilayer preparation at 22ºC with 100 mM NaCl 10mM HEPES buffer. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. An equilibrium dissociation constant of 1.28±1.01 µM was determined and a saturation pixel change of -1.28±0.19 pixels. GABA Neutron Reflectometry Neutron reflectometry experiments were performed on the MAGIK detector at NCNR from September 19 to September 24, 2013. A tBLM with 70:30 POPC:POPG was assembled in a flow cell with a volume of 6mL. Initially the bilayer reached 87% completions, so a further 6mL of 70:30 POPC:POPG vesicles were flowed into the flow cell and ruptured using osmotic shock, resulting in an approximately 100% bilayer completion. A baseline reflectometry measurement was performed on the neat bilayer using H2O and D2O buffers of 100mM NaCL and 10mM NaH2PO4. Further neutron reflectometry measurements were performed on 100nM, 1 μM and 1mM GABA concentrations in both H2O and D2O buffers, plus an additional rinse. The data were then fitted to determine the cross-sectional area, in 1/Å2, as a function of distance from the gold substrate in Å (see Figure 9 to Figure 12). GABA was observed to associate with the hydrocarbon tails of the outer leaflets with increasing affinity as the neurotransmitter concentration increased. The depth of the distribution of GABA into the hydrocarbon region and the thickness of the bilayers were there calculated (see Table 3). As the neurotransmitter concentration increased, the thickness of the bilayer decreased and the depth of the GABA penetration increased. Rinsing the bilayer did not result in the removal of the GABA, suggesting that the binding is irreversible. Figure 6: Cross-sectional area 100nM GABA Data for 100nM GABA fitted to show the crosssectional area as a function of height above the substrate. GABA, red, shows a weak affinity to the hydrocarbon chains of the outer leaflet of the bilayer, dark blue. Figure 7: Cross-sectional area 1μM GABA Data for 1μM GABA fitted to show the crosssectional area as a function of height above the substrate. GABA, red, shows a weak affinity to the hydrocarbon chains of the outer leaflet of the bilayer, dark blue. Figure 8: Cross-sectional area 1mM GABA Data for 1mM GABA fitted to show the crosssectional area as a function of height above the substrate. GABA, red, shows a weak affinity to the hydrocarbon chains of the outer leaflet of the bilayer, dark blue. Figure 9: Cross-sectional area post-rinse GABA Data for GABA after rinsing with buffer fitted to show the cross-sectional area as a function of height above the substrate. GABA, red, shows an association to the head groups of the lipids, light blue, suggesting that adsorption is irreversible. GABA Concentration Neurotransmitter volume (Å3/Å2) Neurotransmitter Penetration (Å) Thickness Change (Å) 1 μM 100 nM 1 mM Rinse 0.82-0.31 +0.52 0.56-0.24 +0.42 0.91-0.36 +0.46 0.94-0.41 +0.50 -0.51-9.95 +4.47 -1.52-5.6 +2.9 -4.41-8.48 +0.06 -2.23-8.56 +2.86 -0.12-0.30 +0.19 -0.14-0.15 +0.15 -0.58-0.19 +0.17 -0.96-0.26 +0.16 Table 3: Parameter values obtained from neutron data analysis for GABA. The error bars were obtained using Monte-Carlo resampling technique and give 68% confidence on the parameters. The neurotransmitter location represents the distance from the surface of the hydrocarbon region that the center of mass of the neurotransmitter peak is located; a negative value represents a penetration into the hydrocarbon region while a positive value represents association outside of the hydrocarbons. Acetylcholine Chloride Control A gold surface coated with a SAM from a 70:30 ethanolic solution of βmercaptoethanol and HC18 tether molecules was exposed to acetylcholine chloride concentrations between 10nM and 400mM (see Figure 13). As for GABA, there is no observable change of the SPR signal when adding up to 1mM of acetylcholine. For concentrations higher than 1mM an increase of the SPR signal that is strictly linear with acetylcholine concentration is observed. This linear increase does not show saturation and can be attributed exclusively to a change of the reflective index of the bulk solvent rather than to surface-bound acetylcholine. As for GABA, this linear relation between the change of the bulk refractive index and the SPR signal will be used to distinguish between surface-bound GABA and change of the bulk solvent refractive index at high acetylcholine concentrations in the following experiments using lipid bilayers. For this correction, a linear function was subtracted that had been determined by fitting the data points between 5.5mM and 100mM. The correction is valid up to 100mM and above this concentration, the control curve is nonlinear with respect to the concentration of AcCh (see Figure 14). Figure 13: Sample 6, no lipid bilayer - SAM only, Acetylcholine Chloride. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. There is no binding to the SAM in the low concentration region up to 1 mM Acetylcholine Chloride. Above 1 mM Acetylcholine Chloride a continuous change of the refractive index of the bulk solution due to the high concentration of Acetylcholine Chloride is observed. This signal is used as a correction for samples 4 and 5. Figure 14: Sample 6, no lipid bilayer - SAM only, Acetylcholine Chloride. Red: SPR raw signal (pixel change), blue: corrected signal by subtracting the high-concentration slope determined from the data point from 5.5 103 µM to 105 µM. The correction is valid up to 105 µM and the corrected curve deviates less than 1 pixel from zero in this range. Acetylcholine Chloride Three experiments with acetylcholine were performed by exposing a tBLM composed of 70:30 POPC:POPG to neurotransmitter concentrations between 10nM and 5.5mM (see Figure 15), and between 10nM and 400mM (see Figure 16 and Figure 17). None of these experiments showed any neurotransmitter binding between 10nM and 5.5mM. However, all experiments showed a decrease of the SPR signal by less than 1 pixel over this range. The second and the third experiment, which were extended to higher acetylcholine concentrations, did show the characteristic increase of the SPR signal at high concentrations due to changes of the bulk refractive index. However, this region could not be fit to a linear function. Therefore in the second experiment, the whole binding curve was fit to a single sigmoidal absorption isotherm with a KD in the high-concentration region plus a linear function that models the high-concentration refractive index increase. This model function is able to fit the data only when a negative saturation pixel change is used. The fit values are KD=15.6±6.2mM and Bmax=-8.8±2.2 pixel. The negative saturation value is consistent with the decrease of the SPR signal at lower concentrations observed in both experiments. It might be interpreted as an adsorption of acetylcholine to the bilayer that results in a substantial bilayer thinning. In order to study the region of about 10 mM in more detail, a higher measurement point density was used in that region during the third experiment. Figure 17 displays a difference plot of the binding curve shown in Figure 18 and a linear function. Before and after the feature that deviates from a linear function, the difference is a constant. The feature itself is composed of an initial increase in the SPR signal and a later decrease that levels out at a net decrease of the signal. Therefore, the second and the third acetylcholine experiments are in agreement with each other. Further, the increase of the SPR signal due to the associated mass of acetylcholine at the bilayer and the decrease of the SPR signal due to the (presumed) membrane thinning are offset. A first attempt of fitting the difference signal also showed that both processes probably cannot be described by single-adsorption Langmuir isotherms. Figure 15: Sample 4, 70:30 POPC:POPG, Acetylcholine Chloride, bilayer preparation at 40ºC. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. Coefficient values ± one standard deviation offset =0.7 ± 0.127 kd =15576 ± 6.19e+03 bmax =-8.8088 ± 2.11 slope =0.00056992 ± 1.67e-05 concoffset =1 ± 0 SPR pixel change 100 10 1 0.1 10 -2 -1 10 10 0 1 10 2 10 concentration / µM 10 3 4 10 10 5 Figure 16: Sample 5, 70:30 POPC:POPG, Acetylcholine Chloride, bilayer preparation at 40ºC. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. The binding curve was fitted to a single sigmoidal function plus a linear function of the concentration. Obtained fit results are KD=15.6±6.2mM and Bmax=-8.8±2.2 pixel. Figure 17: Sample 8, 70:30 POPC:POPG, Acetylcholine Chloride, bilayer preparation at 40ºC. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. The binding curve was fitted to a linear function of the concentration. Clearly visible are deviations from the linear function at around 104 µM. Figure 18: Subtraction of a linear function from the binding curve of sample 8. Before and after the feature around 104 µM the difference is a constant. The two enveloping red curves are sigmoidal functions in analogy to a binding curve with parameters KD=8000 µM, and Bmax values of 5 and -2.6. Serotonin Results A single experiment with serotonin was performed by exposing a tBLM composed of 70:30 POPC:POPG to neurotransmitter concentration between 10nM and 10mM (see Figure 19). This experiment did not show any neurotransmitter binding between 10nM and 100uM, showing a decrease of the SPR signal of 1.9 pixels over this range. Above 100μM, the SPR pixel value increased as the bulk concentration of the neurotransmitter increased. As with the acetylcholine experiment, the absorption curve for the serotonin was fitted to a sigmoidal absorption isotherm and a negative saturation index increase. The values for 1mM and 10mM concentration of serotonin were excluded from the fit due to their being a higher concentration of bulk serotonin at these two concentrations. The fit values are KD=0.08±0.02μM and Bmax=-2.1±0.1 pixel. The negative saturation value is consistent with the decrease of the SPR signal at lower concentrations observed in the experiment, and may be interpreted as the adsorption of serotonin to the bilayer resulting in bilayer thinning. Figure 19: Sample 10, 70:30 POPC:POPG, Serotonin hydrochloride, bilayer preparation at 22ºC. Top: SPR raw data, pixel change vs. time. Bottom: Binding curve extracted from equilibrium pixel changes from SPR raw data. The binding curve was fitted with a Langmuir isotherm with a negative saturation value. Clearly visible are deviations from the curve around 100μM. Serotonin Neutron Reflectometry After rinsing the flow cell used for the GABA neutron reflectometry experiment above with 100mM NaCL and 10mM NaH2PO4 in H2O and D2O buffers, serotonin experiments were performed in the same flow cell. Three concentrations of serotonin, 10 nM, 100nM and 10μM, were each prepared in 6mL of H2O and D2O buffers of 100mM NaCL and 10mM NaH2PO4. Reflectometry measurements were then performed on each of these concentrations of serotonin. The results of which were then fitted to determine the cross-sectional area, in 1/Å2, as a function of distance from the gold substrate in Å (see Figure 19 to Figure 22). Serotonin showed a greater association with the bilayer than GABA. The thickness of the bilayer and location of the serotonin distribution was then calculated from this data (see Table 4). Serotonin showed a greater affinity to the bilayer than GABA as demonstrated by the higher values of protein volume per bilayer area. Furthermore, the distribution of serotonin moved towards the center of the bilayer as the concentration of neurotransmitter increased, resulting in a broad distribution at the highest concentration. Figure 20: Cross-sectional area 10 nM Serotonin Data for 10nM serotonin fitted to show the cross-sectional area as a function of height above the substrate. Serotonin, red, shows a weak affinity to the hydrocarbon core of the bilayer, dark blue. Figure 21: Cross-sectional area 100nM Serotonin Data for 100nM serotonin fitted to show the cross-sectional area as a function of height above the substrate. Serotonin, red, shows a weak affinity to the hydrocarbon core of the bilayer, dark blue. Figure 10: Cross-sectional area 100μM Serotonin Data for 100μM serotonin fitted to show the cross-sectional area as a function of height above the substrate. Serotonin, red, shows an affinity to the hydrocarbon core of the bilayer, dark blue. Serotonin Concentration Neurotransmitter volume (Å3/Å2) Neurotransmitter Penetration (Å) Thickness Change (Å) 10 nM 0.40-0.22 +0.40 -8.15-5.63+4.23 -0.09-0.13+0.16 100 nM 0.52-0.21 +0.33 -11.52-3.55 +5.08 -0.34-0.15+0.15 100 μM 0.86-0.20 +0.25 -18.80-1.44 +5.94 -0.35-0.19+0.17 Table 4: Parameter values obtained from neutron data analysis for serotonin. The error bars were obtained using Monte-Carlo resampling technique and give 68% confidence on the parameters. The neurotransmitter location represents the distance from the surface of the hydrocarbon region that the center of mass of the neurotransmitter peak is located; a negative value represents a penetration into the hydrocarbon region while a positive value represents association outside of the hydrocarbons. Discussion Comparison to Wang, C. et al. In comparison to the publication “Wang, C. et al., 2011. Journal Of Physical Chemistry B, 115(1), pp.196–203”, which uses membranes composed of 90:10 DMPC/DMPG, the present work uses a membrane composition of 70:30 POPC/POPG. See Figure 11 for a reproduction of figure 3 from Wang et al. that contains the measured concentrationdependent binding affinities. Figure 11: Reproduction of Figure 3 from: Wang, C. et al., 2011. Affinity of four polar neurotransmitters for lipid bilayer membranes. Journal Of Physical Chemistry B, 115(1), pp.196–203. Wang et al. find membrane affinities for GABA and acetylcholine that depend linearly on their concentration in the bulk medium. Affinities Γ3 approximate number of neurotransmitters per lipid and range between 0.01 and 0.08 over a concentration range of 5mM to 40mM for both, GABA and acetylcholine. These affinity values are smaller than the estimates of the maximum surface coverage obtained with GABA in this work which ranges between 1 and 5 lipid molecules per neurotransmitter or Γ3 values between 0.2 and 1. In complete disagreement with Wang et al. is the obtained dissociation constant K D of GABA binding to POPC/POPG. We found a KD of about 1 µM and did not see any further adsorption of GABA up to a concentration of 100 mM. The estimate of K D≈10mM for acetylcholine that was obtained in the present work, does agree with the observed binding of Wang et al. in the region between 5mM and 40mM. However, in the present work we observe saturation at about 40mM, for which there is no indication in the data from Wang et al. Further Experiments With the present data, the bilayer thinning observed with acetylcholine is still a hypothesis. Neutron reflectivity experiment should be able to verify bilayer thinning. One would have to measure three subsequent conditions: the neat bilayer, the bilayer in contact with NT, and rinse. We certainly want to extend the experiments with PG lipids to PS lipids. Discussion Notes Notes from a discussion with Robert Cantor – Summer 2013 1. The low KD values for GABA and serotonin are still surprising and ideally need confirmation by another technique. In principal NR is such a technique, but Fred Lanni’s experiments on the influence of NT on the bilayer undulations of red blood cells might another such experiment. 2. The second biggest issue is the lack of reversibility seen in the NR experiments upon rinsing of the system. One should check on this with SPR and consider alternate model systems, like solid supported membranes. A general bias of the tBLMs towards insertion would have far-reaching consequences. 3. The SPR data in Figure 4 of GABA at a 70:30 POPC:POPG membrane, appears to have another binding at 1 mM. It might be interesting to experimentally follow up on this. 4. The magnetic reference layer technique should prove useful to confirm and repeat the NR data. 5. There were GUV micro-pipetting experiments with NTs. They might give insight into binding affinities - check literature. 6. Consider measuring NT partitioning into bilayers using the Nagle’s equipment. 7. Do we have access to fluorine NMR experiments and could we get kinetic information from them. Notes from a discussion with Vitalii Silin – Summer 2013 In general the results are convincing, but there are a number of things that would strengthen the work: (1) The linear response of the SPR signal with respect to NT concentration for both control experiments should be backed up with refractive index measurements of a series of NT concentrations. One should find the same limit to linearity in those measurements than in the SPR measurements. (2) The SPR measurements miss a rinsing step at the end of each concentration series. This would be necessary to check for reversibility of the NT binding. One could also find arguments to perform a rinsing step after each incubation with NT. (3) If one would like to discern bilayer thinning and NT association, as it might be important for acetylcholine, a stopped flow setup might be helpful. The dissociation step in this setup might give extra information. Notes from a discussion with Robert Cantor – Summer 2013 Robert remains skeptical towards the measured low KD of GABA and he looks for a potential explanation of that result. He states that such a low K D would not be consistent with his kinetic model of GABA-membrane interaction. A few future experiments were identified: 1. Repeat the GABA experiment with 10% POPG instead of 30%. This repeat would match the bilayer condition that Peter West was using. 2. Glycine is expected to behave similar to GABA because it is also zwitterionic. The downside is that Glycine is even smaller than GABA. 3. Neutron experiments for acetylcholine and GABA. 4. Experiments with serotonin.