Phosphorus
advertisement

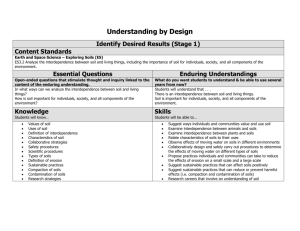
Phosphorus - P
o Phosphorus levels are low in soils- in archeology P enrichment is evidence of human influence
o Phosphorus can lead to pollution- P from sewage sludge and agricultural lands
Phosphorus in plant Nutrition
o Essential component of ATP (.. energy currency):
for uptake of nutrients
transport within plants
assimilation into different biomolecules: DNA, RNA, Phospholipids
(important in cellular membranes); bones and teeth
o Plant tissue contains 0.2-0.4% P in DM
o Important for - photosynthesis
-Nitrogen fixation
-Flowering fruiting and seed production,
-maturation, crop quality and structural strength
Deficiency Symptoms
o Stunting, thin stems
o Dark green foliage (bluish green)
o Delayed maturity, sparse flowering, poor seed quality
o Yellowing and senescence of leaves
o Purpling in some plants in leaves and stems
o Mobile in plants therefore older leaves show deficiency first
The phosphorus problem in soil
o Soil P levels are low (200-2000kg/Ha to 15cm depth
o Soil P compounds are largely insoluble and unavailable
o Phosphorus is fixed and rendered unavailable in soils- even that applied as manure and
fertilizer
1
o Lack of P is usually the first limiting factor in crop production.
Land degradation
o As a result of too little P in soils
o Common in many highly weathered soils (humid and sub-humid regions)
-
intense weathering- induced P losses and low availability Al-Phosphorus
compounds dominate these soils
-
P cycling reserves P in soils as long as no disturbances occur to the system
-
Damage results from clearing of forests which increases P losses in R/off
o Soil P levels decline and re-growth of vegetation bcmes sparse:
-
Leguminous N fixation is impeded
-
Erosion accelerates
-
WHC declines
-
Soils are increasingly impoverished and there is progressively less vegetation
cover
-
Soil degradation is accelerated
Water quality degradation
o is promoted by – agric soils are enriched in P from livestock wastes, P in run off and P from
fertilized lands.
o There is excessive eutrophication from point sources and non-point sources
o Point sources- outflow from sewage effluent and industrial plants
o Non-point sources- Run off water and sediments from scattered soils though out the affected
watershed
o Agric operations increase soil P levels and surface run-off and therefore P to streams
o Animal manures have high P levels from P-enriched feeds
o Downstream eutrophication occurs
o Run off increased by surface tillage increases particulate P losses in sediments
o R/Off also carries dissolved P esp when manures are left on surface unincorporated
2
o Tillage disturbances tend to promote loss of clay and OM fractions which are relatively rich in
P leaving behind the coarser fractions which are lower in P levels.
The Phosphorus cycle
o Effective P management in soils only possible with an understanding of the various pools of
soil P and the interactions between them
P in Solution
o Very low- 0.001-1 mg/L
o Forms taken up by plants are strongly pH dependent
H2PO4- { - monovalent - predominates at pH = 4 to 4.5}
HPO42- {- divalent - predominates under alkaline conditions}
Soluble org Phosphorus
Both divalent and monovalent present at pH around neutral
HPO42-
H2PO4-
H3PO4
PO43-
100
50
Concentration %
2
4
6
8
10
12
14
pH of solution
o Uptake by plants is aided by mychorrhizal fungi
Fungal hyphae solubilize and intercept P making it available to plants
Fungal hyphae form a dense network.
Decomposition of plant residues
3
o P assimilated by plants may be released during mineralization
o Immobilization by microbes follows and may lead to formation of various pools of organic
Phosphorus- active, passive and slow pools
o Microbial death and subsequent mineralization results in repletion of the cycle
Soil Phosphorus forms
o Plant available P is usually < 0.01% of P total in soil
o Phosphorus is present in soil as :
i)
Organic Phosphorus –Phosphate esters (inositol phosphates); nucleic acids and
phospholipids
ii)
Calcium Phosphate copds (in alkaline soils)
iii)
Iron- and aluminium-P inorganic compounds (in acid soils)
o All three forms of phosphorus are insoluble and only contribute slowly to soil solution
inorganic P.
Organic P- mineralization & immobilization
immobilization
Fe2+, Al3+, Ca2+
Microbes
Org P
H2PO4
-
Fe2+, Al3+, Ca2+ Phosphates
microbes
insoluble
mineralization
o Immobilization: C: P> 300: 1
o Mineralization: C:P < 200: 1
o Organic P contributes more to plant Phosphorus needs in intensely weathered soils like oxisols
ultisols ( rich in Fe and Al oxides that are extremely insoluble)
Inorganic P
o Mainly in 2 groups: Ca—P compounds and Al, Fe or Mn—P compounds (calcium phosphate
compounds or aluminium—, Iron— or Manganese—phosphate compounds
o Ca—P compounds:
more soluble as pH decreases.
Therefore disappear in acid soils.
4
Apatite minerals are the least soluble
o Mn—P compounds:
Iron and aluminium hydroxy-phasphates: eg strengite and variscite.
Have very low solubility in acid soils. Bcme more soluble as pH increases.
Therefore unstable in alkaline soils but predominant in acid soils.
o Aging:- Ca—P compounds and Fe—P compounds bcme less and less soluble with time ie
freshly fixed P may be more soluble and its solubility declines to low level with time.
o
This concept is used to remove waste water and sewage sludge
Al3+ + H2PO4- + 2 H2O
2H+ + Al(OH)2 H2PO4
Soluble
insoluble
o Fe, Al oxides and 1:1 clays may fix large quantities of phosphorus by these reactions
ie H2PO4- reacts with and becomes adsorbed to surfaces of insoluble oxides of iron aluminium
and manganese e.g. gibbsite (Al2O3.3H20) and goethite (Fe2O3.3H20)
o Oxides may be coatings on clay particles and in interlayer surfaces.
o A series of reactions lead to occluded P whereby H2PO4- becomes the integral part of oxide
minerals—and is buried inside the oxide… hence the aging phenomenon.
Iron reduction in wet soils-effect on P availability
o Prolonged anaerobic (anoxic) conditions can lead to the reduction of Iron III to iron II (ie Fe3+
to Fe2+).
o When this happens the Fe—P complex becomes more soluble and P is released into solution.
o →This is important in improving the availability of P in paddy rice fields.
o → Thus Phosphorus bound as part of sediments in streams and river beds may become soluble
and cause eutrophication in the future .. even after the erosion and sediment loss has been put
under control.
Phosphorus in high pH soils
o Similar rxns happen under alkaline conditions
CaCO3
Ca(H2PO4).H2O + 2 H2O
Monocalcium phosphate
(soluble)
→
2(CaHPO4.2H2O) + CO2↑
Dicalcium phosphate
(slightly soluble)
5
→
Ca3H(PO4)2 +CO2↑ + 5H2O
Tricalcium phosphate
(very low solubility)
o NB as fertilizer H2PO4- is added to alkaline soils, a series of rxns change H2PO4- to mocalcium
phosphate, dicalcium phosphate and the tricalcium phosphate.
o The solubility of tricalcium phosphate declines further with the formation of hydroxyl, oxy
carbonate and flouroapatite.
o Bacteria and fungi release citric acid which can increase the solubility of Ca—P and Al—P
copds for their own use but this eventually bcmes available to plants.
P fixing capacity of soils
Change in soln P
(Quantity factor, Q)
P retained mg P/Kg soil
P removed
Eqm P concentration in solution (Mg/L)
(INTENSITY FACTOR I)
o Can be determined using a known quantity of soil
o Equilbrated with solution of varying concentrations successively
o P will be adsorbed and a certain amount of P will remain in solution- this is the eqm
phosphorus concentration (EPC).
o EPC0 is the EPc of a solution that gives zero adsorption of P
o Desorption can also occur
o The relations shows the balance between intensity and capacity factors in soil fertility
o PBC =
Q
I
,
PBC is the potential buffering capacity
6
o Importance : lies in that the relationship gives an indication of
i)
ii)
potential for P losses in R/O
capacity of soil to supply P as it is removed by plants
Factors affecting P fixation in soils
o P fixing capacity- dependent on clay type and clay content
2:1 clays<<1:1 clays < carbonate xstals< xstalline Al, Fe, Mn oxides< armophous Al, Fe, Mn
oxides< allophone
o Soil pH
-
- p fixtion highest at pH extremes i.e. very acid and very alkaline soil conditions
phosphate fixation is lowest at pH = 6 – 7
o Effect of Organic matter:- addition of decomposable OM reduces P fixation
-
OM molecules mask fixation sites and prevent P fixation on those sites
-
Organic matter anions produced during decomposition by plant roots and
microbes compete with P for exchange or fixation sites on clay colloids
-
Chelation of Fe, Mn and Al and their subsequent removal from forming insoluble
P copds
Practical control of P in soils
i)
Quench soil P fixing capacity by adding P in excess of crop requirements
-
Localized placement-banded application of P to reduce costs
-
In no-till systems, P may be broadcast on the surface forming a
horizontal band.
ii)
Combine ammonium and phosphorus fertilizers:
-
ammonium and phosphorus fertilizers are mixed in a band
-
ammonium fertilizers releases acidity when NH4+ oxidizes to NO3-
-
Uptake of excess NH4+ ions also produces acidity which aids
solubilization of P compounds.
iii)
Cycling of organic matter
7
-
microbial break down of OM releases P slowly facilitating uptake by
mycchorrhizae and plants before fixation occurs
-
organic compounds can reduce P fixation capacity of soils
-
Enhanced by the use of P efficient plants which encourage cycling
iv)
Control of soil pH between 6 and 7
v)
Enhance mychorrhizal symbiosis
vi)
-
Include good michorhizal host plants
-
Reduce tillage
-
Inoculation with appropriate fungi
choose efficient plants- some plants require less p in soil solution
-
extensive root systems by monocots
-
N-fixing legumes acidify their rhizosphere by taking little nitrate
-
Some plants secrete such compounds as piscidic acid (pigeon pea)
which complexes Fe thereby increasing availability of Fe—P cpds
-
Plants in the cruciferae family (cabbage, mustard seed) excrete malic
and citric acids and also form extensive root systems.
Choice of plant will aid restorative ecology approaches and initiatives.
8
9