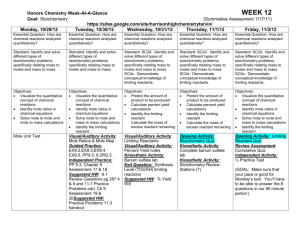
Ch 3 Stoichiometry
advertisement
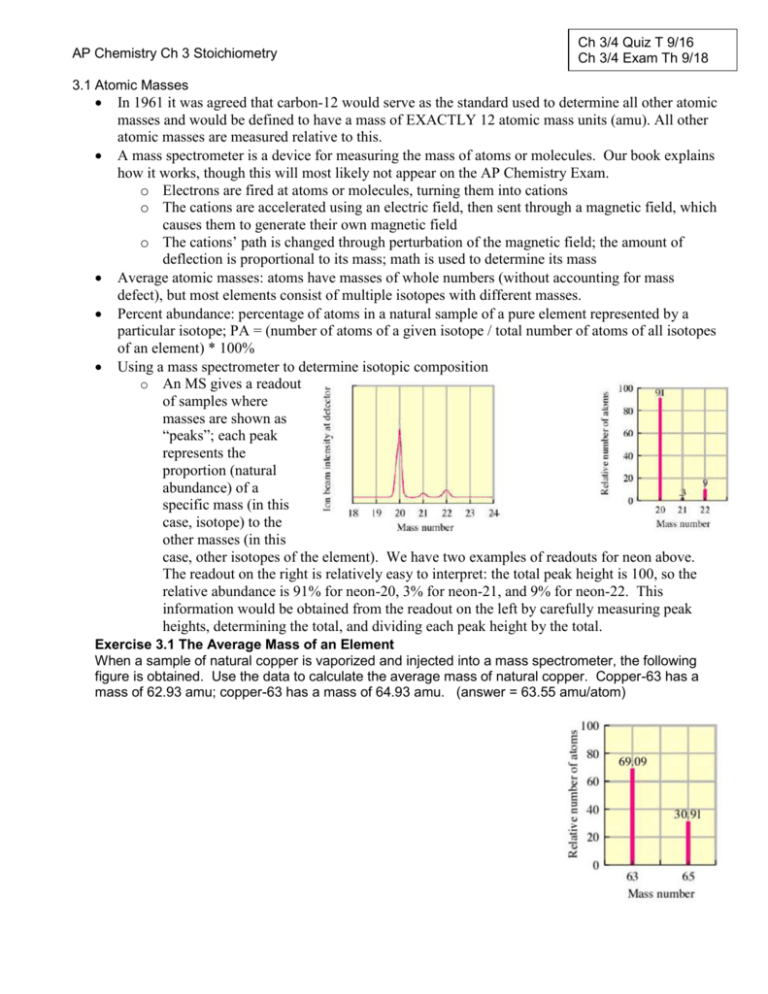
AP Chemistry Ch 3 Stoichiometry Ch 3/4 Quiz T 9/16 Ch 3/4 Exam Th 9/18 3.1 Atomic Masses In 1961 it was agreed that carbon-12 would serve as the standard used to determine all other atomic masses and would be defined to have a mass of EXACTLY 12 atomic mass units (amu). All other atomic masses are measured relative to this. A mass spectrometer is a device for measuring the mass of atoms or molecules. Our book explains how it works, though this will most likely not appear on the AP Chemistry Exam. o Electrons are fired at atoms or molecules, turning them into cations o The cations are accelerated using an electric field, then sent through a magnetic field, which causes them to generate their own magnetic field o The cations’ path is changed through perturbation of the magnetic field; the amount of deflection is proportional to its mass; math is used to determine its mass Average atomic masses: atoms have masses of whole numbers (without accounting for mass defect), but most elements consist of multiple isotopes with different masses. Percent abundance: percentage of atoms in a natural sample of a pure element represented by a particular isotope; PA = (number of atoms of a given isotope / total number of atoms of all isotopes of an element) * 100% Using a mass spectrometer to determine isotopic composition o An MS gives a readout of samples where masses are shown as “peaks”; each peak represents the proportion (natural abundance) of a specific mass (in this case, isotope) to the other masses (in this case, other isotopes of the element). We have two examples of readouts for neon above. The readout on the right is relatively easy to interpret: the total peak height is 100, so the relative abundance is 91% for neon-20, 3% for neon-21, and 9% for neon-22. This information would be obtained from the readout on the left by carefully measuring peak heights, determining the total, and dividing each peak height by the total. Exercise 3.1 The Average Mass of an Element When a sample of natural copper is vaporized and injected into a mass spectrometer, the following figure is obtained. Use the data to calculate the average mass of natural copper. Copper-63 has a mass of 62.93 amu; copper-63 has a mass of 64.93 amu. (answer = 63.55 amu/atom) 3.2 The Mole A mole of any element/compound/etc. contains 6.02 x 1023 particles of it. We refer to this as Avogadro’s number. I prefer using dimensional analysis to solve problems related to moles and stoichiometry, but some of you may be out of practice with this. You might want to use a mole map when you do your homework until you are comfortable with this idea, but you cannot use it on quizzes or tests. Sig figs—stick with 2 decimal places; this generally won’t lead you wrong. Exercise 3.2 Determining the Mass of a Sample of Atoms Americium is an element that does not occur naturally. It can be made in very small amounts in a device called a particle accelerator. Using your periodic table, calculate the mass in grams of six atoms of americium. (answer = 2.42 x 10-21 g Am) Exercise 3.3 Determining Moles and Atoms From Mass Calculate both the number of moles and the number of atoms in a 10.0 g sample of aluminum. (answer = 0.371 moles, 2.23 x 1023 atoms Al) 3.3 Molar Mass and Formula Weight Molar mass (MM) is the sum of all the atomic masses in a given chemical formula in units of g/mole. It is also equal to the mass in grams of one mole of a substance. Empirical formula is the ratio of each type of atom in a chemical formula; it can be the same as the chemical formula but it is not always. o Ex. for an ionic substance, the formula unit such as MgF2 is really the ratio of cations (Mg2+) to anions (F-) o Ex. for a network covalent substance such as silicon dioxide, there are 2 atoms of oxygen for every atom of silicon o Ex. glucose C6H12O6 can be reduced to the empirical formula CH2O Formula weight is a term used for ionic substances; molecular weight is used for covalent substances; molar mass can be used for all Exercise 3.4 Calculating Molar Mass I Juglone is a dye produced from the husks of black walnuts. The formula for juglone is C10H6O3. Calculate the molar mass of juglone. (answer = 174.1 g/mole) Calculate the number of moles in a sample of 1.56 x 10-2 g of pure juglone. (answer = 8.96 x 10-5 mol) Exercise 3.5 Calculating Molar Mass II Calculate the mass in grams of a 4.86 mole sample of calcium carbonate. (answer = 486 g) Calculate the mass of carbonate ions present in this sample. (answer = 292 g carbonate ions) Exercise 3.6 Molar Mass and Numbers of Molecules Calculate the number of carbon atoms present in a 1 μg sample of isopentyl acetate, C7H14O2. (answer = 4 x 1016 carbon atoms) 3.4 Elements that Exist as Molecules Pure hydrogen, nitrogen, oxygen and the halogens exist as DIATOMIC molecules under normal conditions. MEMORIZE!!! Be sure you compute their molar masses as diatomics. We lovingly refer to them as the “gens”, “Hydrogen, oxygen, nitrogen & the halogens!” Alternatively BrINClHOF or HOBr FINCl Others to be aware of, but not memorize: o P4—tetratomic form of elemental phosphorous; an allotrope o S8—sulfur’s elemental form; also an allotrope o Carbon—diamond and graphite covalent networks of atoms 3.5 Percent Composition of Compounds Percent composition by mass o Ex. ethanol, C2H5OH Exercise 3.7 Calculating Mass Percent Penicillin has the formula C14H20N2SO4. Calculate the mass percent of each element. (answers = 53.82%, 6.47%, 8.97%, 10.26%, and 20.49% respectively) 3.6 Determining the Formula of a Compound Calculating empirical and molecular formula: remember that the empirical formula is the mostreduced form of the molecular formula, and that the two are sometimes identical. o When calculating empirical formula, always assumed there are 100 g of a sample unless you are actually given mass. This works if you are given percentages. Hydrate vs anhydrous o Hydrates have water (“dot waters”). This water counts in the determination of molar mass. o Anhydrous substances do not have water. Ex. A compound is composed of carbon, nitrogen and hydrogen. When 0.1156 g of this compound is reacted with oxygen [a.k.a. “burned in air” or “combusted”], 0.1638 g of carbon dioxide and 0.1676 g of water are collected. Determine the empirical formula of the compound. Exercise 3.8 Empirical and Molecular Formulas Determine the empirical and molecular formulas for a compound composed of 71.65% Cl, 24.27% C, and 4.07% H. The molar mass of this compound is 98.96 g. (answers = C4H5N2O and C8H10N4O2) 3.7 Balancing Chemical Equations In a chemical reaction, bonds are broken (requires energy) and new bonds are formed (releases energy). A balanced chemical equation shows the relative amounts of reactants and products by molecule or mole. o State symbols: s, g, l, aq = solid, gas, liquid, aqueous solution respectively o It’s a good idea to begin with the most complicated-looking substance when you balance, and to balance elements last o If you get stuck, balance the most complicated-looking substance o Memorize these Metals (M) + halogens (X) MaXb, then balance H2CO3 CO2 + H2O whenever it is formed; never write carbonic acid as a product because it spontaneously decomposes in an open container Metal carbonates metal oxides + carbon dioxide Exercise 3.9 Balancing Chemical Reactions I Chromium compounds exhibit a variety of bright colors. When solid ammonium dichromate, a vivid orange compound, is ignited, solid chromium (III) oxide, nitrogen gas, and water vapor are created. Write and balance the equation for this reaction. Exercise 3.10 Balancing Chemical Reactions II At 1000°C, ammonia gas reacts with oxygen gas to form gaseous nitric oxide (NO(g)) and water vapor. Write and balance the equation for this reaction. 3.8 Stoichiometric Calculations: Amounts of Reactants and Products Stoichiometry is the study of the quantities of reactants consumed/products created in chemical reactions. It is very important to Chemistry…and it never goes away. Important things o Write correct formulas, or your work and answer will be negatively affected o Calculate correct molar masses or your work and answer will be negatively affected o Write and balance the chemical equation (correctly…you should see a pattern here) o Use a mole ratio obtained from the balanced chemical equation—this is the “heart” of stoichiometry o Use the mole map and/or dimensional analysis to solve the problem o Use correct units, and label whatever substance you’ve calculated the quantity of You’ve done this before. We’re diving right in. Exercise 3.11 Stoichiometry I Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide by forming solid lithium carbonate and liquid water. What mass of gaseous carbon dioxide can be absorbed by 1.00 kg of lithium hydroxide? (answer = 920. G) Exercise 3.12 Stoichiometry II Baking soda is often used as an antacid due to the fact that it neutralizes excess hydrochloric acid secreted by the stomach. This results in the formation of sodium chloride, water, and carbon dioxide. If one gram of baking soda is used, how many grams of hydrochloric acid can be neutralized? 3.9 Calculations Involving a Limiting Reactant In some stoichiometry problems, you are clearly given an excess amount of one or more reactants (as in, “calcium carbonate reacts with an excess amount of hydrochloric acid”, etc.). There are many other problems where you are given two quantities that do not “match up” according to their mole ratio. For these types of problems, you will need to determine which reactant is limiting and which one is present in excess. The limiting reactant controls how much of your product(s) is/are made, as well as how much of each of the excess reactant(s) is/are consumed. A common example is the formation of ammonia gas from nitrogen gas and hydrogen gas. First write the equation: N2 + H2 NH3 Now balance the equation: N2 + 3 H2 2 NH3 You will likely be given quantities for both reactants. These can be given as moles, grams, etc. You know that you need a ratio of 1 mole nitrogen to 3 moles hydrogen, so you need to convert any units you receive to moles (unless they are given in moles, of course). You’ll need the molar masses of both compounds. Diatomic nitrogen = 28.04 g/mole and diatomic hydrogen = 2.02 g/mole. To continue our example, let’s say you have 25.0 kg of nitrogen and 5.0 kg of hydrogen and you need to determine the mass of ammonia that can be produced, as well as which reactant is limiting, and how much of the excess reactant will be left over. Convert nitrogen to moles: Convert hydrogen to moles: There should be three times as many moles of hydrogen as nitrogen. Are there more, or less? Which reactant is limiting? Now use the amount of the limiting reactant to calculate the amount of ammonia we will obtain. Since the reactants were given in kg, we’ll calculate the products in kg. Lastly, we will determine the amount of the excess reactant that is consumed, and subtract it from the original amount to determine how much is left over. I’ve gone through each step separately here, so it looks like a lot of work. This problem also asked three questions; not all limiting reactant problems will do this. Some will skip right to “how much X is formed?” or “how much Y is consumed/leftover?” which leaves you to fill in the individual steps. Exercise 3.13 Limiting Reactant Nitrogen gas can be prepared by passing gaseous ammonia over solid copper (II) oxide at high temperatures. The other products of this reaction are solid copper and water vapor. If a sample containing 18.1 g of ammonia reacts with 90.4 g of Cu), which is the limiting reactant? How many grams of nitrogen will be formed? (answer = 10.6 g N2) 3.10 Yields Theoretical yield is the amount of product formed when a limiting reactant is completely consumed, under perfect conditions. In other words, it’s the maximum amount you could possibly form. Nature is not so kind. Actual yield is…do I need to define this one? Percent yield is the ratio of actual yield to theoretical yield. Exercise 3.14 Theoretical Yield and Percent Yield Methanol (CH3OH) is the simplest alcohol. It can be manufactured by combination of gaseous carbon dioxide and hydrogen. Suppose 68.5 kg CO(g) and 8.60 kg H2(g) react. Calculate the theoretical yield of methanol. (answer = 6.86 x 104 g or 68.6 kg) If 3.57 x 104 g of methanol are actually produced, what is the percent yield of methanol? (answer = 52.0%)