immediately - University of Warwick
advertisement

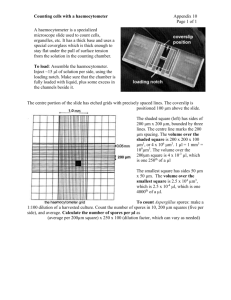
University of Warwick Headstart Summer School Cell Biology Day Haematology Lab Manual Contents 1. Introduction to practical Haematology 2. Safety Issues 3. Aims a. Blood Smears b. Cell counting c. Measuring the Packed Cell Volume (PCV) 4. Using a haemocytometer 5. Reference Values for cell count 1. Introduction to practical haematology The clinical specialty of haematology (aka “blood sciences”) is that branch of medical science that deals with pathological conditions of the blood. The role of the haematology laboratory is to carry out tests whose results, together with clinical features of the patient, will guide the physician in making a diagnosis and planning treatment. There are many different blood disorders and their classification is muddled. Perhaps put most simply the major disorders are those in which there are: Abnormalities of cell number and/or phenotype (morphology, expression of specific proteins, presence of specific genetic defects) such as the anaemias and the leukaemias; Problems with blood clotting (too much or too little) sometimes referred to as coagulopathies: and especially monitoring coagulation in patients treated with anticoagulant drugs e.g. warfarin. The main task of the haematology laboratory is to investigate these disorders. The key techniques are counting cells in samples of patients’ blood and bone marrow, assessing cell phenotypes, and measuring the rate at which blood clots under carefully standardised conditions. Haematology laboratories can also be involved in other disorders, such as the haemoglobinopathies, investigation of some infectious diseases (e.g. malaria) and the monitoring of inflammatory conditions. 2. Safety Issues There is a clear and well-understood risk from blood borne infections when handling human tissues and in particular, blood. On the whole, acute infections (measles, septicaemia etc) are not a problem since the affected individual is aware of them. However there are several chronic infections which may be initially symptomless but have very serious long-term consequences. Of these, the most important are infections by human immunodeficiency virus (HIV), hepatitis B and C viruses (HBV & HCV). These infections are transmitted from person to person by exchange of blood (or other bodily fluids). This can occur when working in a laboratory through, for example, needlestick injuries (i.e. accidentally being stabbed with a syringe needle contaminated with infected blood) or through an open wound being splashed with blood. However, such accidents, given proper care, are very rare. Further, the probability of undergraduates carrying these viruses is extremely small: for example, the prevalence in the UK of HBV is less than 0.5% overall, and the infection tends to be restricted to longterm intravenous drug users and one or two other groups in society. Therefore risk in this class is vanishingly small, and has been assessed as such by the departmental safety committee: furthermore, the Health and Safety Executive do not object to this class. But in order to comply with good practice when working with human material: Sharps including the single use lancets and glass microscope slides must be discarded immediately after use into a yellow-lidded sharps container. Porous material (e.g. tissues or cotton wool) contaminated with blood must be discarded into a ‘hazardous waste’ sack. Disposable plastic materials contaminated with blood or blood products must be disposed of into a hazardous waste scak. Tips and all other materials must be discarded into a ‘disposo-safe’ jar present on the bench. This jar when filled should be discarded in to the hazardous waste sack. All wounds should be covered with a waterproof plaster (including those caused by finger-prick). Discarded plasters must be disposed of in the porous material hazardous waste sack at the end of the days practical. Any blood spilled onto the bench or floor must be cleaned up immediately using disinfectant. If any blood is spilled onto a laboratory coat, this coat must be changed for a clean one. Good laboratory practice must be observed at all times o Laboratory coats must be worn at all times (buttoned up). o Safety glasses should be worn at all times. o Disposable gloves should be worn. o Long hair must be tied back. o Shoes that fully encase the foot must be worn Any careless or flippant behaviour will potentially result in exclusion. 3. Aims The objective of the present class is for students to become familiar with basic haematological techniques: obtaining blood samples and determining cell counts. All students will do all the tasks with their own blood. If a student has any reason why not to do these tests on his or her own blood, please speak to a demonstrator. 3.1 Produce a blood smear and stain using Heamofast staining 3.2 Cell counting with a heamocytometer 3.3 Measure the packed cell volume (PCV: aka haematocrit value). 3.1 Producing a Blood Smear 3.1.1. Taking blood There are two means of sampling blood for routine blood tests: venepuncture for venous blood (which must be carried out by trained professionals), and skin (usually finger) prick, which obtains capillary blood. The finger prick (which we shall use today) is done using an automatic device that requires little training in its use. Materials: Unistik 3 Extra 0.5 mL screw-cap blood-collection tubes containing potassium EDTA Elastoplasts Method: Before taking blood, ensue that the skin to be lanced is CLEAN and DRY. Prick your finger using Unistik 3 Extra (designed to obtain a larger volume of blood) and collect the blood in a green screw top tube which contains heparin as anti-coagulant. Gently massage your finger to encourage bleeding, massaging from the base towards the end. Wipe with a tissue if it starts to clot. Aim to obtain at least a few tens of µL and preferably more than 100 μL. Your partner (wearing gloves) should hold the tube for you, gently tapping it on the bench so that the blood runs down to the bottom. Cap the tube. The blood will remain unclotted and usable for a few hours at room temperature. Bear in mind you have ten fingers: if you do not get enough blood from the first, go for the other 9. 3.1.2 Making blood smears Materials: Glass slides Blood sample Microhaematocrit capillary or Gilson pipette and tips Method: This will be demonstrated. Make several smears, you will need AT LEAST 3 but make more in case you need to repeat any of the stainings. Using a microhaematocrit capillary tube, pick up a very small volume of blood and place this on a (labelled) slide, cleaned in alcohol, about 1cm from the edge of the frosting. A drop about 5mm across is needed. Take another slide, touch it to the first at an angle of about 30°, bring it to the drop of blood and allow the drop to spread across the edge of the 2 nd slide, then draw the 2nd slide smoothly along to smear out the drop into a thin film. Dry the films immediately by waving the slide in air. 3.2 Cell Counting with a Haemocytometer A full (or complete) blood cell count (FBC) gives the numbers of red cells, white cells and platelets. An absolute white blood cells count gives the number of white blood cells and a differential count gives the numbers of subsets of white cells. A red blood cell count (RBC) gives numbers of red blood cells. Counts will be done with a haemocytometer Materials: Haemocytometer Pipette and tips Erythrocyte lysis buffer A (2% acetic acid/gentian violet) for WBC Erythrocyte lysis buffer B (1% ammonium oxalate) for platelets Phosphate buffered saline (PBS) 1.5 ml tubes Rack Method: Load the haemocytometer carefully to ensure the chamber is not flooded. Leave on the bench for a minute or two to allow the cells to settle, longer for platelets. Count at least 100 cells and preferably 400 for a reliable estimation. More details in Appendix. Absolute WBC: Dilute a sample of the blood 1 in 20 in erythrocyte lysis buffer A (2% acetic acid/gentian violet). Ensure it is well mixed. Leave to stand for at least 2 min, remixing from time to time. (This will lyse the red cells but leave the white cells intact.) Calculate the absolute white blood cell count. Red cell count: Dilute blood 1 in 200 in phosphate buffered saline (PBS) mix well and determine the red cell count. Platelet count: Dilute a sample of the blood 1:20 in erythrocyte lysis buffer B (1% ammonium oxalate) and mix well. Determine the platelet count. Hint: For a 1 in 100 dilution take 1μl of blood and add 99μl of appropriate buffer Cell count type Number (cells per μl) Absolute WBC Red Blood Cell Count Platelet Count 3.3 Measuring Packed Cell Volume (PCV) When investigating red blood cells, cell number is not the only important parameter: cell volume is another. The packed cell volume (PCV) is the volume percentage % of red blood cells in the total blood volume. Materials: Blood sample Microhaematocrit capillary tubes and wax for sealing tubes Centrifuge. Method: Ask for a demonstration before proceeding Take up blood into microhaematocrit tube by capillarity, leaving a few mm of the tube empty. Seal the tube with was (check with a demonstrator that it is sufficiently sealed) and centrifuge for 5 minutes at full speed. Once spun down, PCV can be calculated by measuring the length of the red blood cells and dividing it by the total length of blood cells and expressing this as a percentage. Calculate the PCV PCV (the volume percentage % of red blood cells in the blood) 4: Counting cells using a haemocytometer 4.1 The Haemocytometer: As its name implies, the HAEMOCYTOMETER is a device originally designed to count blood cells, but it may be used to count any microscopic particle including all sorts of cells. Use of the haemocytometer allows the determination of the total number of organisms in a suspension, whether living or dead. Electronic particle counters may also be used e.g. the Coulter Counter. The number of living cells must be determined by other methods (viable counts :Reference Section 10). The haemocytometer is a large microscope slide with a counting chamber 0.1 mm deep formed by placing a glass coverslip of the top of the central depression of the slide. On the bottom of the chamber is an etched square divided into 9 squares each of area 1 mm2. The central square is divided into 25 smaller squares and each of these are subdivided into 16 squares (see figure 9.1). The principle of its use is that a suspension of cells is introduced into the counting chamber, and the number of cells over a defined area is counted: knowing the depth and area gives the volume and so the number of cells in that volume. 4.2 Cell counting: Slightly dampen the surface of the slide by breathing on it and fix the coverslip over the chamber by firmly pressing at its edges. Take the cell suspension (WELL MIXED - this is very important as the cells settle out quickly) and place a drop on the edge of the coverslip: surface tension should draw the suspension into the chamber. Do not flood the slide nor allow the coverslip to float away! Check with the microscope: almost certainly there will be vast numbers of cells, impossible to count, so dilute the suspension until there are moderate numbers of cells visible. Record the dilution factor! The counting of the cells depends on their size. Bacteria or erythrocytes, are usually counted by counting the cells in the central block of 25 small squares: count all the cells in the 5 squares indicated by shading in the diagram, multiply by 5 to obtain the total per 1 mm2. Other cells are counted in the 4 squares, subdivided into 16, located at the corners of the chamber. Count all the cells in one of these to obtain number per 1 mm2. Simple arithmetic shows that the volume over a 1 mm square is 0.0001 ml. Hence the number of cells in that square, multiplied by 10,000, gives the number of cells per ml of the diluted suspension. Cells lying on the etched lines should not be counted twice. A convention to avoid this is to count cells lying on the bottom and right hand lines (as you view the slide) ignoring those on top and left lines. Any counting operation is subject to random error. You should therefore always count a sufficient number of cells to ensure your result is not significantly affected by this. A rule of thumb is to count a minimum of 100 cells, if necessary by counting over more squares than is suggested above. Fig 2: the haemocytometer grid. Figure 3: the haemocytometer grid. For rbc count, count all rbcs in 5 of the central 25 squares (as shown) to estimate cell count Figure 4: the haemocytometer grid. For wbc count, count all wbcs in the squares circled and average 5. Reference Values for number of blood cells Table 1 gives the reference ranges (the term “normal values” is now obsolete) for the main cell types. The” reference range” is obtained by carrying out measurements on a large number of individuals and placing them in rank order: values 47.5 percentiles on either side of the median, which include 95% of the total, comprise the reference range. If the data are normally distributed this is approximately 2 standard deviations on either side of the mean. Table 1. Reference ranges* for blood cells Cell type erythrocytes total white blood cells lymphocytes neutrophils monocytes platelets Reference range** (cells per µL)*** (%) 4-6 x 106 (PCV man: = 0.45 +/- 0.05; woman: 0.41 +/- 0.05) 4-11 x 103 1.3-4.0 x 103 (20-40%) 2-8 x 103 (40-60%) 0.2-0.8 x 103 (~5%) 1-4 x 105 *Approximate, for adults. Males and females differ slightly: neonates and infants have markedly different values (see Dacie & Lewis Practical Hematology (ed Lewis SM, Bain BJ & Bates I) 10th edn Churchill Livingstone Elsevier, Philadelphia 2006 pp 16-18 for values for children aged 0-12y). **Go to http://en.wikipedia.org/wiki/Reference_ranges_for_blood_tests#Hematology for reference values for a wider range of haematological parameters. ***Values are also often quoted per L which of course are 106 times bigger. Note that μL and mm3 are the same thing.