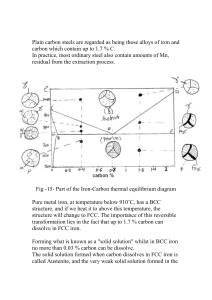
Influence microalloying elements
advertisement

Influence of the microalloying elements on the temporary inhibition of static recrystallization by strain-induced precipitates Manuel GÓMEZ1)*, Alberto QUISPE2), Sebastián F. MEDINA1) 1 National Centre for Metallurgical Research, CENIM-CSIC, Av. Gregorio del Amo 8; 28040 Madrid, Spain. 2 National University “Jorge Basadre”, Av. Miraflores s/n, University City, Tacna, Peru. *Corresponding author: E-mail: mgomez@cenim.csic.es 1 The kinetics of static recrystallization of austenite and its transitory inhibition by strain-induced precipitates have been characterized in several microalloyed steels with different compositions. This inhibition can be seen by the formation of “plateaus” in the curves of static recrystallization obtained from isothermal double-deformation tests. The influence of the type of microalloying element (Nb, V, Al) and the mean size of the precipitates on the duration time of the plateau of recrystallization inhibition has been studied and empirical relationships between these variables have been obtained. Alsteels present a much coarser particle size and a considerably shorter plateau compared to Nb and V-microalloyed steels. KEY WORDS: Microalloyed steel; Austenite; Static recrystallization; Precipitation; Kinetics; Plateau of recrystallization inhibition 2 1. Introduction The amount and type of microalloying elements play an important role on the shape and the nature of nanoprecipitates formed in microalloyed steels. The elements most typically considered as microalloying elements are Ti, Nb and V, although Al is frequently considered as a microalloying element as well. At equal level of alloying, the precipitates of the microalloying elements are soluble in austenite as follows[1]: Ti<Al<Nb<V, and their carbides are usually more soluble than their nitrides. The static recrystallization is different before and after strain-induced precipitation. At higher temperatures, when elements are in solution, the recrystallization kinetics of austenite can be described by an Avrami equation[2]: t X a = 1 - exp - 0.693 t 0.5 n (1) where Xa is the recrystallized volume fraction and t0.5 is the time corresponding to 50% recrystallization, which depends on all the major variables that intervene in hot deformation and whose most general expression is: t0.5 A p q D s exp Qx RT (2) where is the strain applied, the strain rate, D the austenite grain size, Qx the activation energy for recrystallization, T the absolute temperature, R=8.3145 Jmol-1K-1, and p, q and s are parameters. While p and q are negative values, s is positive. Under certain conditions of deformation and below a critical temperature, strain-induced precipitation starts and the recrystallization kinetics cannot be described only by Eq. 1. The fine precipitates formed by the microalloying elements and interstitials (C, N) exert a pinning force on austenite grain boundaries in motion. As a result, recrystallization is temporarily inhibited and a horizontal “plateau” appears in the curves.[3-5] After longer 3 times, precipitates coarsen, so pinning forces are again lower than recrystallization driving forces, the plateau ends and the value of Xa grows again.[6-9] Few works have previously focused on the quantitative influence of the type of microalloying on the duration time of the plateaus. This work presents empirical equations that illustrate the strong variation in the extent of temporary blockage of recrystallization for Nb, V and Al microalloyed steels. 2. Experimental Data related to kinetics of static recrystallization and strain-induced precipitation from more than twenty steels with different compositions were collected and analyzed. The steels contained a range of combinations of C, N and single or complex additions of precipitate-forming elements such as V, Nb, Al and Ti (Table 1). Most of the steels were manufactured by Electroslag Remelting (ESR) in a laboratory unit capable of producing 30 kg ingots. Recrystallization and precipitation were studied by means of isothermal hot torsion tests using specimens with a gauge length of 50 mm and a diameter of 6 mm. Before deformation, the specimens were austenitized. The reheating temperature varied according to composition and was set to be higher than the solubility temperature in order to completely dissolve precipitates. Ti-added steels represented an exception, as the low solubility of TiN precipitates in austenite makes it difficult or even impossible to reach complete dissolution.[1,10] After austenitization, the specimens were rapidly cooled to the deformation temperature in order to prevent precipitation prior to deformation. The deformation temperatures were between 1150ºC and 800ºC and the recrystallized fraction was determined for several post-deformation holding times. The double deformation technique [11,12] was used to calculate Xa, in particular applying the 4 method known as “back extrapolation”.[13,14] The accuracy of this method has been verified by comparing with metallographic observations.[4] Strain rate varied between 0.91 and 3.63 s-1. The applied strains of 0.20 and 0.35 were insufficient to promote dynamic recrystallization. It is known that critical strain for dynamic recrystallization is slightly lower than peak strain (p). Empirical expressions published elsewhere [15,16] allowed to calculate p as a function of initial grain size (D0), Zener Hollomon parameter (Z), activation energy (Q) and composition. It was confirmed that the values of strain applied remained below the critical strain, as the calculated values of p were much higher than (most of them between 0.5-1.5). This was also confirmed by observing the values of critical strain found by other authors for similar steels and strain rates [17]. Phase transformation temperature Ar3 was determined by dilatometry tests. Table 2 summarizes the testing conditions, solubility temperatures[1] and Ar3 temperatures for the steels studied. Carbon extraction replica technique was used to study the precipitation state by means of transmission electron microscopy (TEM). 3. Results and Discussion The torsion test gives the values of torque applied versus the number of turns made on the specimen, which are transformed respectively into equivalent stress and strain using Von Mises criterion.[18] Fig. 1a shows an example of the evolution of Xa versus the time after deformation for a V-microalloyed steel. The progress of recrystallization following Avrami´s law before and after the formation of the plateau of inhibition of recrystallization caused by the pinning effect of precipitates (as described above) can be seen in the figure. Fig. 1b shows another example of recrystallization kinetics for a Nb-microalloyed steel. In this 5 particular case, two successive plateaus were distinguished. It has been previously described that the formation of two types of precipitates with similar stoichiometries and precipitation temperatures can facilitate the formation of two plateaus in Nb and Vmicroalloyed steels.[7,19,20] Curves like those shown in Fig. 1 where the plateau is well defined can be used to deduce the temperatures and times corresponding to different recrystallized fractions. The points that define the start and the end of the plateau are taken to plot the induced precipitation start (Ps) and finish (Pf) curves, respectively. In this way recrystallization–precipitation–time–temperature (RPTT) diagrams can be drawn.[6] The value of Xa does not vary between the Ps and Pf curves and is represented by a horizontal line. Once the Pf curve is reached, the lines of each Xa drop again. Fig. 2 shows examples of RPTT diagrams of a V and an Al-microalloyed steel. In general, the length of the plateaus in curves as those shown in Fig. 1 is longer for the case of Nb-microalloyed steels, compared to V-steels. Al-microalloyed steels usually present the shortest plateaus of recrystallization inhibition[6] or even no plateaus.[21] This can be also observed comparing the time interval (distance) between Ps and Pf curves in RPTT diagrams like those shown in Fig. 2. In steels where Ti is the sole microalloying addition, the occurrence of plateaus in recrystallization curves is possible[22] but less frequent. This is due to the high solubility temperature of TiN that restricts the strain induced precipitation. If strain induced precipitation does not take place or if it is insignificant, static recrystallisation follows the sigmoidal law versus time continuously.[23] Similar to recrystallization, the strain induced precipitation occurring mostly during the time interval of the plateau can be supposed to obey Avrami's law[24], and the precipitated fraction (Xp) can be expressed as: 6 n t X p 1 exp ln 0.95 t0.05 (3) If Xp = 0.95 in Eq. (3), the following expression may be deduced: 1/ n ln 0.05 t0.95 ln 0.95 t0.05 (4) The times to reach 5% and 95% of precipitated volume fraction (t0.05 and t0.95, respectively) can be assumed to coincide approximately with tN and t'N, the times corresponding to the nose of the Ps and Pf curves, respectively.[4] Fig. 3 shows the values of t0.95 as a function of t0.05 for all the steels studied. It can be seen that the steels can be classified in three distinct groups according to the microalloying element. The regression of the values of t0.05 and t0.95 for the different categories of microalloyed steels gives the following equations: Nb and Nb-Ti microalloyed steels: t0.95 14 .15t0.05 1.0001 (5) V and V-Ti steels: t0.95 7.26 t0.05 0.9999 (6) Al steels: t0.95 2.1t0.05 1.02 (7) Eqs. (5) to (7) confirm that strain-induced precipitation follows Avrami's law, as in all cases the exponent found for the parameter t0.05 is close to 1, which agrees with Eq. 7 4. Previous figures and Eqs. (5)-(7) also show that the length or duration of the plateau of recrystallization inhibition is not constant, but it is a function of the type of microalloying element and follows the order: Nb>V>Al. The much shorter length of the plateaus for Al-microalloyed steels can be well explained by the considerably coarser sizes of AlN precipitates compared to other strain-induced precipitates, as seen in Fig. 4. It is known that the pinning forces exerted by precipitates decrease for lower precipitated volume fractions and coarser sizes.[6,22] The coarser size of AlN particles results from the larger diffusion coefficient of Al compared to Nb and V, as well as the higher precipitation temperature of AlN.[23] For similar reasons, it has been found that Nb(C,N) particles nucleate earlier and grow faster than VN particles.[19] However, the plateaus in Nb-microalloyed steels are longer than for the case of V-steels. This means that, apart from the mean particle size (considering similar levels of precipitated volume), other factors might have an effect on the duration of the inhibition of recrystallization by precipitates. It has been previously suggested that the highest effectiveness of Nb to inhibit static recrystallization comes from the smaller effect of V in solution (solute drag) compared to Nb[14,25], but results presented in this work correspond to times and temperatures were strain-induced precipitation is taking place. Another possible explanation could be sought on the differences in the interaction between precipitates and grain boundaries in Nb and V-steels. On this regard, other alloying elements such as Mn and Mo can modify the activity coefficients of microalloying and interstitial elements and the solubility of precipitates.[26] Consequently, this can exert a complex influence on the retardation of the start and the end of precipitation and on the extent of the temporary inhibition of recrystallization. 8 Finally, it must be taken into account that Nb precipitates have higher solubility temperatures than V precipitates (as seen in Table 2) and the same can be said for the nose temperatures. At higher precipitation temperatures diffusion is faster and supersaturation is lower, so it can be expected that the balance between growth and nucleation rates will be different in Nb precipitates and V precipitates. V particles (formed at lower temperatures) will nucleate faster and will grow less than Nb particles, which explains their lower average size. However, it has been previously found [27] that Nb microalloyed steels can present a bimodal distribution of precipitate sizes at the end of the plateaus, with an important amount of very fine precipitates. This can be explained by the more sluggish nucleation at higher temperatures that allows to have a significant population of “new” and relatively fine precipitates capable of inhibiting recrystallization after long post-deformation times, despite the coarser average size of Nb particles compared to V precipitates. 4. Conclusions The extent of the temporary inhibition of recrystallization by strain-induced precipitates is a function of microalloying element. Empirical equations obtained from the study of more than twenty steels confirm that strain-induced precipitation kinetics obeys Avrami's law and show that the duration of the plateau in the curves of recrystallization kinetics follows the order: Nb>V>Al. When single additions of Ti are employed, the formation of plateaus associated to strain-induced precipitation is difficult because of the low solubility of TiN. These results have important implications on the evolution of microstructure during and at the end of thermomechanical processing of microalloyed steels and help to 9 explain why Nb is the most adequate element to obtain unrecrystallized austenite at the end of hot rolling. Acknowledgements The authors gratefully acknowledge the financial support of Spanish Ministry of Economy and Competitiveness thorough the project ref. MAT2011-29039-C02-02. 10 References [1] E.T. Turkdogan, Iron Steelmaker 1989, 16, 61. [2] J.H. Beynon, C.M. Sellars, ISIJ Int. 1992, 32, 359. [3] M.J. Luton, R. Dorvel, R.A. Petkovic, Metall. Trans. A 1980, 11, 411. [4] A. Quispe, S.F. Medina, M. Gomez, J.I. Chaves, Mater. Sci. Eng. A 2007, 447, 11. [5] S. Vervynckt, K. Verbeken, P. Thibaux, Y. Houbaert, Steel Research Int. 2011, 82, 369. [6] M. Gómez, L. Rancel, S.F. Medina, Mater. Sci. Eng. A 2009, 506, 165. [7] M. Gomez, S.F. Medina, J.I. Chaves, Mater. Sci. Forum 2007, 550, 417. [8] Y. Cao, F. Xiao, G. Qiao, C. Huang, X. Zhang, Z. Wu, B. Liao, Mater. Sci. Eng. A 2012, 552, 502. [9] S. Vervynckt, K. Verbeken, P. Thibaux, Y. Houbaert, Mater. Sci. Eng. A 2011, 528, 5519. [10] P.E. Reynolds, Ironmaking Steelmaking 1991, 8, 52. [11] S. Gelder, B. Buchmayr, B. Linzer, G. Hohenbichler, Steel Research Int. 2011, 82, 1213. [12] B. Niznik, R. Kuziak, M. Pietrzyk, Steel Research Int. 2012, 83, 743. [13] J.S. Perttula, L.P. Karjalainen, Mater. Sci. Technol. 1998, 14, 626. [14] H.L. Andrade, M.G. Akben, J.J. Jonas, Metall. Trans. A 1983, 14, 1967. [15] S.F. Medina, C.A. Hernández, Acta Mater. 1996, 44, 137. [16] S.F. Medina, C.A. Hernández, Acta Mater. 1996, 44, 149. [17] C. Ghosh, V.V. Basabe, J.J. Jonas, Steel Research Int. 2013, 84, 490. [18] A. Faessel, Rev. Métall. Cah. Inf. Tech. 1976, 33, 875. [19] S.F. Medina, M. Gomez, P.P. Gomez, J. Mater. Sci. 2010, 45, 5553. 11 [20] H.S. Zurob, C.R. Hutchinson, Y. Brechet, G.R. Purdy, Mater. Sci. Eng. A 2004, 382, 64. [21] P. P. Suikkanen, V. T. E. Lang, M. C. Somani, D. A. Porter, L. P. Karjalainen, ISIJ Int. 2012, 52, 471. [22] M.I. Vega, S.F. Medina, A. Quispe, M. Gómez, P.P. Gómez, ISIJ Int. 2005, 45, 1878. [23] S.F. Medina, M. Gomez, P. Valles, Steel Res. Int. 2010, 81, 1010. [24] Z. Wang, Q. Yong, X. Sun, Z. Yang, Z. LI, C. Zhang, Y. Weng, ISIJ Int. 2012, 52, 1661. [25] M.G. Akben, I. Weiss, J.J. Jonas, Acta Metall. 1981, 29, 111. [26] M.G. Akben, B. Bacroix, J.J. Jonas, Acta Metall. 1983, 31, 161. [27] S. F. Medina, A. Quispe, P. Valles, J. L. Baños, ISIJ Int. 1999, 39, 913. 12 (a) (b) Fig. 1. Recrystallized fraction (Xa) versus time (t). (a) V-microalloyed steel; (b) Nbmicroalloyed steel. 13 (a) (b) Fig. 2. RPTT diagrams. (a) V-microalloyed steel; (b) Al-microalloyed steel. 14 Fig. 3. Time to reach 95% of precipitated volume fraction (t0.95) as a function of the time for 5% of precipitation (t0.05). 15 (a) (b) Fig. 4. TEM images of carbon replicas. (a) Nb carbonitrides in a 0.040% Nb steel; (b) Al nitrides (coarse rectangles) and VN precipitates (fine particles) in a 0.05% V0.02% Al steel. 16 Table 1. Chemical compositions of steels studied [mass %]. Steel C Si Mn Al V Nb Ti N V1 0.11 0.24 1.1 0.012 0.043 0.0105 V2 0.12 0.24 1.1 0.012 0.060 0.0123 V3 0.11 0.24 1.0 0.01 0.093 0.0144 V4 0.21 0.2 1.1 0.009 0.062 0.0134 V5 0.33 0.22 1.24 0.011 0.076 0.0146 V6 0.35 0.21 1.23 0.008 0.033 0.0121 V7 0.42 0.24 1.32 0.012 0.075 0.02 V8 0.37 0.24 1.42 0.012 0.120 0.019 TV1 0.55 0.29 1.06 TV2 0.34 0.22 1.08 0.009 N1 0.11 0.24 1.23 0.002 0.041 0.0112 N2 0.11 0.24 1.32 0.002 0.093 0.0119 N3 0.21 0.18 1.08 0.007 0.024 0.0058 N4 0.21 0.19 1.14 0.008 0.058 0.0061 N5 0.51 0.25 1.2 0.008 0.026 0.0105 N7 0.29 0.22 1.3 0.006 0.066 0.0062 N8 0.20 0.2 1.0 0.006 0.007 0.0056 N9 0.46 0.24 1.25 0.011 0.009 0.01 NT1 0.21 0.22 1.18 0.007 0.028 U7 0.095 0.321 1.525 0.029 0.003 Y1 0.099 0.297 1.463 0.037 0.002 Y7 0.102 0.284 1.479 0.02 0.063 0.019 0.0174 0.055 0.024 0.0182 0.004 0.024 0.006 0.0042 0.01 0.0158 17 Table 2. Testing conditions (reheating temperature, initial austenite grain size, strain, strain rate), solubility temperatures[1] and phase transformation temperature Ar3 for the steels studied. Solubility temperatures (ºC) Steel V1 V2 V3 V4 V5 V6 V7 V8 TV1 TV2 N1 N2 N3 N4 N5 N7 N8 N9 NT1 U7 Y1 Y7 R.T (ºC) 1230 1230 1100/1230 1100/1200 1200 1200 1200 1200 1200 1200 1230 1230 1250 1250 1275 1295 1250 1250 1250 1200 1200/1300 1200/1300 D (μm) 172 167 125/165 95/180 165 170 162 157 31 53 122 116 210 190 430 415 140 190 55 127 165/550 151/550 0.20/0.35 0.20/0.35 0.20/0.35 0.35 0.20/0.35 0.20/0.35 0.35 0.20/0.35 0.20/0.35 0.35 0.20/0.35 0.20/0.35 0.20/0.35 0.20/0.35 0.35 0.20/0.35 0.20/0.35 0.20/0.35 0.20/0.35 0.20/0.35 0.20/0.35 0.20/0.35 𝜀̇ VC0.75 (s-1) 3.63 730 3.63 758 3.63 785 3.63 791 3.63 833 3.63 773 0.91/3.63 847 3.63 879 3.63 849 1.09/3.63 809 3.63 3.63 3.63 3.63 1.09/3.63 3.63 3.63 3.63 3.63 3.63 3.63 3.63 VN NbC0.87 NbN NbC0.7N0.2 TiC 968 1012 1070 1023 1051 957 1082 1126 1050 1041 719 746 18 TiN AlN 1100 1120 1117 1095 1130 1069 1183 1176 1122 1228 1126 1241 1237 1302 987 1090 1144 878 1151 1232 1054 1129 1107 1141 965 1022 1069 912 1165 1249 1146 1233 1226 1272 1037 1116 1161 953 1207 1505 1174 1530 1133 919 925 976 994 1053 967 957 1084 1114 1438 979 1096 1244 1221 Ar3 (°C) 786 782 784 768 716 715 718 721 693 718 786 786 768 769 674 751 770 704 768 List of Figure Captions. Fig. 1. Recrystallized fraction (Xa) versus time (t). (a) V-microalloyed steel; (b) Nbmicroalloyed steel. Fig. 2. RPTT diagrams. (a) V-microalloyed steel; (b) Al-microalloyed steel. Fig. 3. Time to reach 95% of precipitated volume fraction (t0.95) as a function of the time for 5% of precipitation (t0.05). Fig. 4. TEM images of carbon replicas. (a) Nb carbonitrides in a 0.040% Nb steel; (b) Al nitrides (coarse rectangles) and VN precipitates (fine particles) in a 0.05% V-0.02% Al steel. List of Table Captions. Table 1. Chemical compositions of steels studied [mass %]. Table 2. Testing conditions (reheating temperature, initial austenite grain size, strain, strain rate), solubility temperatures[1] and phase transformation temperature Ar3 for the steels studied. 19