Change in Temperature Lab Teacher
advertisement

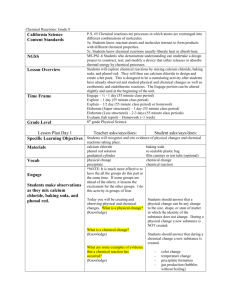
Change in Temperature - Adapted from American Chemical Society (Inquiry in Action) Teacher Copy for Grade 4 Quarter 1 Change in Temperature Lab and Power Point Presentation THIS LAB IS IN CONJUCTION WITH QUARTER 1: SC.4.P.9.1 – CHANGES IN MATTER PPT SC.4.P.9.1 Identify some familiar changes in materials that result in other materials with different characteristics, such as decaying animal or plant matter, burning, rusting, and cooking. Problem Statement: Does changing the amount of calcium chloride being mixed with a baking soda solution decrease, increase, or have no effect on the temperature during their chemical reaction? Hypothesis: Changing the amount of calcium chloride (DampRid) being mixed with baking soda solution will increase/will decrease/have no effect on the temperature during their chemical reaction. Variables: Test (Independent/Manipulate) Variable: amount of calcium chloride (DampRid) Outcome (Dependent/Responding) Variable: Temperature in °C. Controlled (Constants) Variables: same amount of baking soda solution, same environment, same measuring unit, same size cups, same procedures …etc. Materials: Baking soda solution, 100 mL DampRid (calcium chloride) Paper towels 5 mL (1 Teaspoon) 2 Thermometer 2 clear small plastic cups Graduated cylinder, 50 mL 1 blue tray Timer 2 ½ mL (1/2 Teaspoon) Goggles (students should wear them at all time) Teacher Procedures: 1. Prepare baking soda solution: Make the baking soda solution for the entire class by adding 1/2 cup (~120 mL) of baking soda to 4 cups (~950 mL) of water. Stir until the baking soda is as dissolved as possible. It’s ok if some is left undissolved. 2. Divide the class into 4 groups. Assign a trial number to each group and ask them to record the data on their lab sheet for the trial number they were assigned. 3. Collect the data from each group. Each group’s recorder will post their data on a chart paper or promethean board for the class to see. Each group will need to copy the data for each trial on their labs sheets. Student (group) Procedures: 1. Use a graduated cylinder to measure 30 mL of baking soda solution and pour into one clear plastic cup. 2. Place a thermometer in the baking soda solution. Tilt the cup to make sure the bulb of the thermometer is submerged. Observe thermometer in the baking soda solution for 2 minutes; record its temperature in degrees Celsius (°C) on the chart. 3. While the thermometer is in the cup, add 3 teaspoons (15 mL) of DampRid (calcium chloride) and watch the thermometer to observe any change in temperature. Record the highest reaction temperature in °C. Record any other observations on the back of the lab sheet. 4. Get a second cup and repeat steps 2-4 changing the amount of DampRid (calcium chloride) to 4 ½ teaspoons (22 ½ mL). Record other observations on the back of the lab sheet. 5. Repeat steps 2-5 two more times for trials #2, #3, and #4. 6. Average the highest reaction temperature for both amounts of DampRid (calcium chloride). 7. Use your senses to make additional observations and record as part of qualitative data. Department of Science Change in Temperature - Adapted from American Chemical Society (Inquiry in Action) Teacher Copy for Grade 4 Quarter 1 Change in Temperature Lab and Power Point Presentation Trial #1 Baking soda solution temperature (°C) Amount of DampRid (Calcium chloride) in Teaspoon Highest reaction temperature (°C) 21 °C 21 °C 3 tsp 4½ DATA TABLE Trial #2 Trial #3 19°C 3 tsp tsp 35 °C 45 °C 20°C 4½ 20°C 20°C 3 tsp 4½ tsp 40°C 50°C Trial #4 21°C 3 tsp tsp 35°C 45°C Average 19°C 4½ 3 tsp tsp 37°C 46°C 4½ tsp 37 47 DATA: QUALITATIVE (USING YOUR SENSES) OBSERVATION Discuss Qualitative Observations. Additional signs of a chemical change observed could include bubbles and fizzing from the gas being produced and the formation of a solid white substance in the bottom of the cup. The formation of a solid white substance in the bottom of the cup is an example of precipitate which is evidence of a chemical change. Conclusion: Answer the following questions to complete the Conclusion: 1. What was investigated? (State the purpose of the experiment by describing the problem statement.) How will increasing the amount of calcium chloride (DampRid) mixed with baking soda solution affect temperature change in the chemical reaction was investigated. 2. Was your hypothesis supported by the data? (Write a statement as to whether the data supports or does not support the hypothesis including a restatement of the hypothesis.) Answer will vary. Example: The hypothesis was supported (was not supported) by the data. Increasing the amount of calcium chloride (DampRid) will increase the temperature of the chemical reaction. Department of Science Change in Temperature - Adapted from American Chemical Society (Inquiry in Action) Teacher Copy for Grade 4 Quarter 1 Change in Temperature Lab and Power Point Presentation 3. What were the major findings? (Describe the data collected that provides the evidence as to why the hypothesis was supported or not supported.) In all the trials, increasing the amount of calcium chloride (DampRid) increased the temperature of the chemical reaction. The average temperature increase for the four trials was 10°C . Application/Extension: Answer the following questions to complete the Application: 1. How can the investigation be improved? Answers will vary. Watching out for human error by using better accuracy to measure the amount of the substances and/or to read the thermometer. Using cups with a smaller circumference so that the bulb of the thermometer is submerged deeper. Using cups with a smaller circumference so that the bulb of the thermometer is submerged deeper. 2. What are some possible applications of the experiment? (Describe how the findings from this investigation can be used in day-to-day life.) The mixing of calcium chloride (DampRid) and baking soda solution can be used as a source of heat energy to melt ice on roads (de-icer). Increasing the amount of calcium chloride (DampRid) can decrease the amount of humidity in a room faster. 3. What question(s) has your experiment lead you to ask that could be tested in a new investigation? - What effect would changing (increasing or decreasing) the amount of baking soda have on the temperature of a chemical reaction? - Will the rate at which the foam (gas and white precipitate) increase, decrease, or stay the same as a result of increasing the amount of DampRid? Claim Evidence Reasoning – CER – Conclusion Writing Question: Does changing the amount of calcium chloride being mixed with a baking soda solution, increase, decrease, or have no effect on the temperature during the chemical reaction? Claim: (The answer to the question.) Increasing the amount of calcium chloride (DampRid) mixed with baking soda solution increases the temperature of the chemical reaction between the two substances. Evidence: (All the evidence you gathered from hands-on investigations. This includes the observations made and the data collected.) The data collected in the investigation showed the following when the amount of calcium chloride being mixed with a baking soda solution was increased from 3 teaspoons to 4 ½ teaspoons in each of the trials. In trial 1 the temperature increased from 35°C to 50°C. In trial 2 the temperature increased Department of Science Change in Temperature - Adapted from American Chemical Society (Inquiry in Action) Teacher Copy for Grade 4 Quarter 1 Change in Temperature Lab and Power Point Presentation from 40°C to 50°C. In trial 3 the temperature increased from 35°C to 45°C (Record data collected on each additional trial.) The average of all trials showed a temperature increase of 10°C when increasing the amount of calcium chloride from 3 teaspoons to 4 ½ teaspoons. Additional signs of a chemical change observed included bubbles and fizzing from the gas being produced and the formation of a solid white substance in the bottom of the cup. Reasoning: (Why you think the answer is correct. The reasoning explains how the evidence helps answer the question.) An increase in temperature is a sign of a chemical change. The results from the investigation support the claim that increasing the amount of calcium chloride (DampRid) mixed with baking soda solution will increase the temperature of the chemical reaction. In all of the trials the effect of increasing the amount of calcium chloride while keeping the amount of baking soda solution the same increased the temperature of the reaction an average of _____ °C. This evidence confirms that changing the test variable when mixing substances can affect the temperature of the chemical reaction. Department of Science