Set3ans
advertisement

CHE425: Problem set #3
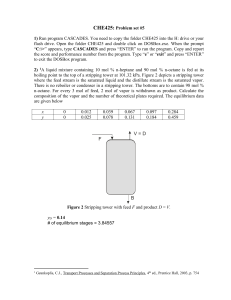
1. Consider the equilibrium stage with C components shown below. Conduct a degree-offreedom analysis by performing the following steps: (a) list and count the variables; (b) write
and count the equations relating the variables; and (c) calculate the degree of freedom.
Equilibrium liquid
from another stage
Exit equilibrium vapor
V, TV, PV, yi
Feed vapor
Feed liquid
Exit equilibrium
liquid phase
LI, TLI, PLI, xiI
Equilibrium vapor
from another stage
Exit equilibrium
liquid phase II
Equilibrium stage
Q
Heat to
(+) or from (-)
the stage
Analysis:
(a)
LII, TLII, PLII, xiII
Number of variables = 7C + 22
(b) The equations are:
Total number of equations = NE = 3C + 12
(c) Degrees of freedom = 4C +10
2. Consider an adiabatic equilibrium flash shown below with all the indicated variables. (a)
Determine the number of variables. (b) Write all the independent equations that relate the
variables. (c) Determine the number of equations. (d) Determine the number of degrees of
freedom. (e) What variables would you prefer to specify in order to solve an adiabatic-flash
problem?
hV, V, TV, PV, yi
Flash drum
Liquid feed
hF, F, TF, PF, zi
hL, L, TL, PL, xi
Analysis:
a) Variables are those appearing in Figure 4.10a
NV = 3C + 9
(b) C Component material balances
(c) NE = 2C + 6
(d) ND = C + 3
(e) Specify the feed completely ( feed rate, temperature, pressure and C - 1
mole fractions) plus exiting pressure.
3) Determine the number of degrees of freedom for a nonadiabatic equilibrium flash for the
liquid feed and three products shown below.
V, TV, PV, yi
Flash drum
Vapor
Liquid feed
Liquid I
LI, TLI, PLI, xiI
F, TF, PF, zi
Liquid II
II
II
II
II
L , TL , PL , xi
Number of degrees of freedom = C + 4
4. For the seven-phase equilibrium system shown below, assume air consists of N2, O2, and
argon. What is the number of degrees of freedom? What variables might be specified? Note:
all species exist in all phases.
Air
n-hexane-rich liquid
Aniline-rich liquid
Water-rich liquid
Phosphorous liquid
Gallium liquid
Mercury liquid
Number of degrees of freedom = 4
Specify T, P, and mole fractions of argon and oxygen in the air.
5.2 A liquid mixture containing 25 mol% benzene and 75 mol% ethyl alcohol, in which
components are miscible in all proportions, is heated at a constant pressure of 1 atm from
60oC to 90oC. Using the spline command in Matlab to plot the following T-x-y
experimental data:
y, x = mole fraction of benzene in vapor and liquid phase, respectively
T,oC 78.4 77.5 75
72.5 70
68.5 67.7 68.5 72.5
y
0
0.075 0.28 0.42 0.54 0.60 0.68 0.73 0.82
x
0
0.015 0.05 0.12 0.22 0.31 0.68 0.81 0.91
75
0.88
0.95
Note: the following Matlab codes can plot the data using spline command:
T=[78.4 77.5 75 72.5 70
68.5 67.7 68.5 72.5 75
77.5
y=[0 0.075 0.28 0.42 0.54 0.60 0.68 0.73 0.82 0.88 0.95
x=[0 0.015 0.05 0.12 0.22 0.31 0.68 0.81 0.91 0.95 0.98
ppx=spline(x,T);
ppy=spline(y,T);
xp=0:0.02:1;yp=xp;
Tx=ppval(ppx,xp);
Ty=ppval(ppy,yp);
plot(xp,Tx,yp,Ty)
grid on
Title('Txy data for benzene-ethyl alcohol at 1 atm')
xlabel('x,y: mole fraction benzene')
ylabel('T(^oC)')
77.5
0.95
0.98
80.1
1.0
1.0
80.1];
1.0];
1.0];
Determine: (a) the temperature where vaporization begins; (b) the composition of the first
bubble of vapor; (c) the composition of the residual liquid when 25 mol% has evaporated,
assuming that all vapor formed is retained in the apparatus and is in equilibrium with the
residual liquid. (d) Repeat part (c) for 90 mol% vaporized. (e) Repeat part (d) if, after 25
mol% is vaporized as in part (c), the vapor formed is removed and an additional 35 mol% is
vaporized by the same technique used in part (c).
Analysis: See plot of T-x-y data on next page, as drawn with a spreadsheet. Curved (instead
of straight) lines connecting the points would be a good improvement.
(a) For a benzene mole fraction of 0.25, a vertical line from M intersects the liquid
line at N at 69.4oC, which is the bubble point.
(b) The benzene mole fraction in the vapor at 69.4oC, obtained from the left-most
vapor line at P, is 0.56.
(c) To find the benzene mole fraction in the liquid at 25 mol% vaporization, extend a
dashed, vertical line upward from the bubble point at N, as shown in the figure on the next
page, until point B is reached. At this point, using the inverse lever-arm rule, the ratio of the
AB line length to the BC line length is 25/75. The benzene mole fraction in the equilibrium
liquid at point A is 0.175 at a temperature of 71.2oC.
(d) To find the benzene mole fraction in the liquid at 90 mol% vaporization, extend a dashed,
vertical line upward from the bubble point, as shown in the figure on the next page, until
point E is reached. At this point, using the inverse lever-arm rule, the ratio of the DE line
length
to the EF line length is 90/10. The benzene mole fraction in the equilibrium liquid at point D
is 0.045 at a temperature of 75.1oC.
(e) To find the benzene mole fraction in the liquid when the liquid from part (c) is
removed from the vapor and further vaporized, proceed as follows. If we start with 100
moles, then the liquid at 25 mol% vaporized is 75 moles with a benzene mole fraction of
0.17. If an additional 35 mol% is vaporized, we will have 35 moles of vapor in equilibrium
with 40 moles of liquid. Therefore, extend a dashed, vertical line upward from point A, as
shown in the figure on the next page, until point H is reached. At this point, using the inverse
lever-arm rule, the ratio of the GH line length to the HI line length is 35/40. The benzene
mole fraction in the liquid at G is 0.05 at a temperature of 74.9oC.
6.2 Stearic acid is steam distilled at 200oC in a direct-fired still. Steam is introduced into the
molten acid in small bubbles, and the acid in the vapor phase leaving the still has a partial
pressure equal to 70% of the vapor pressure of pure stearic acid at 200oC. Use Matlab to plot
the kg acid distilled per kg steam added as a function of total pressure from 101.3 kPa to 3.3
kPa at 200oC. Label the plot with your name using the Title command. The vapor pressure
of stearic acid at 200oC is 0.40 kPa.
P, kPa
101.3
75
50
25
15
10
5
3.3
kg A/kg B
0.0438
0.0592
0.0890
0.1790
0.3006
0.4553
0.9376
1.4650
7. The relative volatility, , of benzene to toluene at 1 atm is 2.5. Construct x-y and T- x-y
diagrams for this system at 1 atm. On the same diagrams, plot the data obtained using the
following vapor pressure data and ideal solution:
TA, TB = temperature of benzene and toluene at the given vapor pressure, respectively
P, torr
20
40
60
100
200
400
760
1,520
O
TA, C
7.6
15.4
26.1
42.2
60.6
80.1
2.6
O
T B, C
18.4
31.8
40.3
51.9
69.5
89.5
110.6
136
Use the diagram for the following: (a) A liquid containing 70 mol% benzene and 30 mol%
toluene is heated in a container at 1 atm until 25 mol% of the original liquid is evaporated.
Determine the temperature. The phases are then separated mechanically, and the vapors
condensed. Determine the composition of the condensed vapor and the liquid residue. (b)
Calculate and plot the K-values as a function of temperature at 1 atm.
Note: Use Matlab to plot the diagrams and label the figures with your name using the Title
command.
T, oC Ps of A, torr Ps of B, torr
80.1
82.5
85.0
87.5
90.0
92.5
95.0
97.5
100.0
102.5
105.0
107.5
110.0
110.5
759.9
817.4
880.8
948.0
1019.1
1094.1
1173.4
1256.9
1345.0
1437.6
1535.0
1637.3
1744.7
1766.8
290.0
314.9
342.7
372.5
404.4
438.5
474.9
513.7
555.2
599.3
646.2
696.1
749.1
760.1
KA
KB
xA
yA
0.9998
1.0755
1.1590
1.2474
1.3409
1.4396
1.5439
1.6539
1.7697
1.8916
2.0198
2.1544
2.2957
2.3248
0.3816
0.4144
0.4510
0.4901
0.5321
0.5769
0.6249
0.6760
0.7305
0.7885
0.8503
0.9159
0.9856
1.0001
1.000
0.886
0.775
0.673
0.579
0.490
0.408
0.331
0.259
0.192
0.128
0.068
0.011
0.000
1.000
0.953
0.899
0.840
0.776
0.706
0.630
0.548
0.459
0.363
0.259
0.146
0.025
0.000
(a) To find the temperature at 25 mol% vaporization, starting with a liquid
mixture of 70 mol% benzene and 30 mol% toluene, extend a dashed, vertical line upward
from point M on the T-y-x diagram on the previous page until point B is reached. At this
point, using the inverse lever-arm rule, the ratio of the AB line length to the BC line length is
25/75. The temperature is 88oC. The benzene mole fraction of the equilibrium vapor when
condensed is the same as the equilibrium vapor at point C or 0.88. The benzene mole
fraction in the residue liquid is the same as the equilibrium liquid at point A or 0.65.
(b) The Raoult's law K-values are included in the above table, and are plotted
below.
8. Saturated-liquid feed of F = 40 mol/h, containing 50 mol% A and B, is supplied to the
apparatus in the following figure.
Vapor
V
Condenser
Feed
F
Heat
Still pot
Reflux
R
Distillate
D
Bottoms
W
The condensate is split so that reflux/condensate = 1. (a) If heat is supplied such that W = 30
mol/h and relative volatility = 2, what will be the composition of the overhead and the
bottoms product. (b) If the operation is changed so that no condensate is returned to the still
pot and W = 3D, compute the product compositions.
xA = 0.4575, xB = 0.5425 for the bottoms
yA = 0.6275, yB = 0.3725 for the distillate
(b) Note that the solution to Part (a) was independent of the reflux ratio. Accordingly, the
solution to Part (b) is the same as for Part (a)
Ref: J. D. Seader and E. J. Henley, Separation Process Principles , Wiley, 2011