Day 100 Assessment Post-HSCT
advertisement
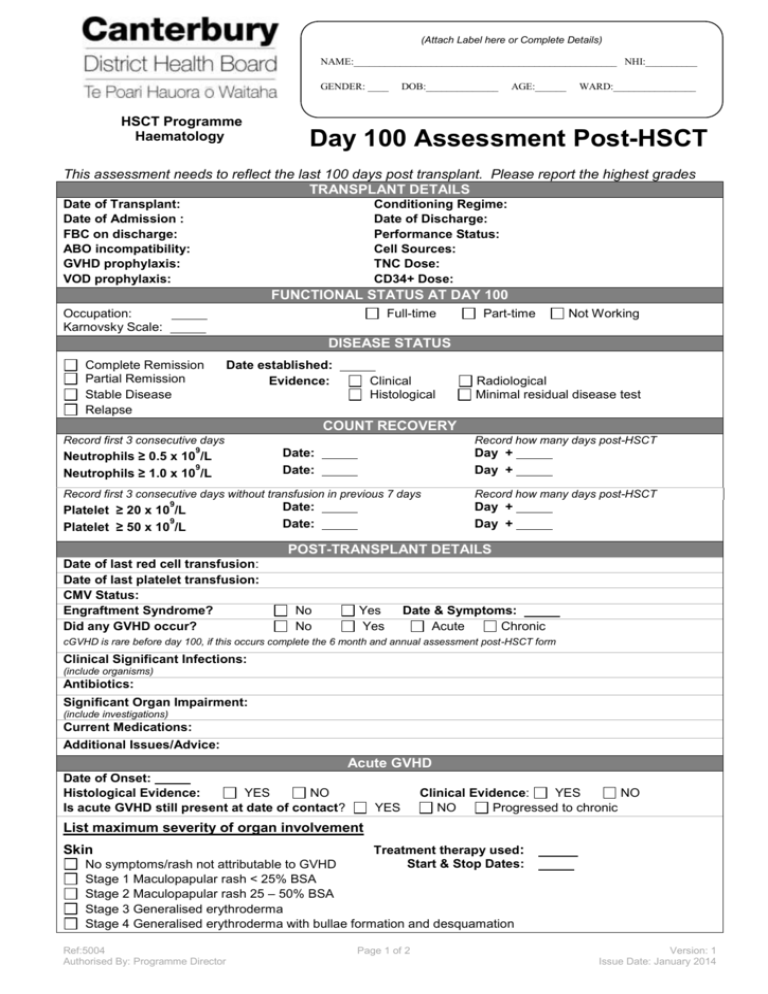
(Attach Label here or Complete Details) NAME:___________________________________________________ NHI:__________ GENDER: ____ HSCT Programme Haematology DOB:______________ AGE:______ WARD:________________ Day 100 Assessment Post-HSCT This assessment needs to reflect the last 100 days post transplant. Please report the highest grades TRANSPLANT DETAILS Date of Transplant: Date of Admission : FBC on discharge: ABO incompatibility: GVHD prophylaxis: VOD prophylaxis: Conditioning Regime: Date of Discharge: Performance Status: Cell Sources: TNC Dose: CD34+ Dose: FUNCTIONAL STATUS AT DAY 100 Occupation: Karnovsky Scale: Full-time Part-time Not Working DISEASE STATUS Complete Remission Partial Remission Stable Disease Relapse Date established: Evidence: Clinical Histological Radiological Minimal residual disease test COUNT RECOVERY Record first 3 consecutive days 9 Neutrophils ≥ 0.5 x 10 /L 9 Neutrophils ≥ 1.0 x 10 /L Record how many days post-HSCT Date: Date: Day + Day + Record first 3 consecutive days without transfusion in previous 7 days 9 Platelet ≥ 20 x 10 /L 9 Platelet ≥ 50 x 10 /L Date: Date: Record how many days post-HSCT Day + Day + POST-TRANSPLANT DETAILS Date of last red cell transfusion: Date of last platelet transfusion: CMV Status: Engraftment Syndrome? Did any GVHD occur? No No Yes Yes Date & Symptoms: Acute Chronic cGVHD is rare before day 100, if this occurs complete the 6 month and annual assessment post-HSCT form Clinical Significant Infections: (include organisms) Antibiotics: Significant Organ Impairment: (include investigations) Current Medications: Additional Issues/Advice: Acute GVHD Date of Onset: Histological Evidence: YES NO Is acute GVHD still present at date of contact? YES Clinical Evidence: YES NO NO Progressed to chronic List maximum severity of organ involvement Skin Treatment therapy used: Start & Stop Dates: No symptoms/rash not attributable to GVHD Stage 1 Maculopapular rash < 25% BSA Stage 2 Maculopapular rash 25 – 50% BSA Stage 3 Generalised erythroderma Stage 4 Generalised erythroderma with bullae formation and desquamation Ref:5004 Authorised By: Programme Director Page 1 of 2 Version: 1 Issue Date: January 2014 (Attach Label here or Complete Details) NAME:___________________________________________________ NHI:__________ GENDER: ____ DOB:______________ AGE:______ WARD:________________ Day 100 Assessment Post-HSCT Treatment therapy used: Gut (lower intestinal tract) Start & Stop Dates: Symptoms not attributable to GVHD Stage 0 No diarrhoea Stage 1 Abdominal pain without significant weight loss (<5%). Diarrhoea ≥ 500ml/day but ≤ 1000ml/ day Stage 2 Mild to moderate weight loss (5-15%) Diarrhoea > 1000ml.day but ≤ 1500ml/day Stage 3 Significant weight loss > 15% Diarrhoea > 1500ml/day Stage 4 Severe abdominal pain with or without ileus Treatment therapy used: Upper Intestinal Tract Stage 0 No persistent nausea or vomiting Stage 1 Persistent nausea or vomiting Start & Stop Dates: Treatment therapy used: Liver No liver acute GVHD / bilirubin level not attributable to acute GVHD Start & Stop Dates: Stage 0 Bilirubin < 2.0 mg/dL (< 34 µmol/L) Stage 1 Bilirubin 2.0 – 3.0 mg/dL (34 – 52 µmol/L) Stage 2 Bilirubin 3.1 – 6.0 mg/dL (53 – 103 µmol/L) Stage 3 Bilirubin 6.1 – 15.0 mg/dL (104 – 256 µmol/L) Stage 4 Bilirubin > 15.0 mg/dL (> 256 µmol/L) Other Organ Involvement: No Yes Organ Involved: Date of Onset: Maximum overall grade (Glucksberg): Grade I (mild) skin stage 1 or 2 only (no liver or gut aGVHD) Grade II (moderate) up to stage 1 liver or gut, up to stage 3 skin Grade III (severe) up to stage 4 liver, up to stage 3 in any other organ Grade IV (life threatening) stage 4 skin or gut, ECOG/WHO performance status 4 or Karnofsky performance score < 30% Physician Performing Assessment Name: Signed: Date: Place form in patients medical record AND email a copy to the BMT Coordinator or fax (03) 364 1486 References: www.bloodref.com Ref:5004 Authorised By: Programme Director Page 2 of 2 Version: 1 Issue Date: January 2014











