Type 3 Deiodinase and Inactivation of Thyroid Hormones
advertisement

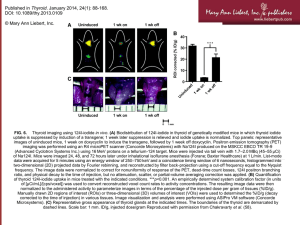
type I idodthyronine deiodinase subunit = Dio1 Three different types of deiodinases are primarily responsible for activation and inactivation of the thyroid hormones L-thyroxine and 3,5,3'-triiodo-L-thyronine. These deiodinases are selenoenzymes that contain selenocysteine as a key active site residue. Type I deiodinase (this enzyme) can catalyze both 5'-deiodination (outer ring) and 5-deiodination (inner ring) of a variety of iodothyronines (including those shown here) and their derivatives (such as sulfate derivatives, see thyroid hormone metabolism II (via conjugation and/or degradation)). It has been found in liver, kidney, thyroid and pituitary. type II iododthyronine deiodinase efficiently catalyzes 5'-deiodination of L-thyroxine to 3,5,3'-triiodo-L-thyronine, an activating reaction. This reaction contributes to systemic and serum 3,5,3'-triiodo-L-thyronine levels. Although L-thyroxine is the major secreted product of the thyroid gland, 3,5,3'-triiodo-L-thyronine is the major bioactive hormone and most circulating 3,5,3'triiodo-L-thyronine is generated by type II deiodinase in peripheral tissues (in [Moreno94]). It also catalyzes 5'-deiodination of 3,3',5'-triiodo-L-thyronine (reverse triiodothyronine). It has been found in pituitary, brain, brown adipose tissue and in human thyroid, skeletal muscle, aortic smooth muscle cells and osteoblasts. type III iododthyronine deiodinase produces inactive or less active metabolites by catalyzing the 5-deiodination of L-thyroxine to 3,3',5'-triiodo-L-thyronine (reverse triiodothyronine), and the 5-deiodination of 3,5,3'-triiodo-L-thyronine to 3,3'-diiodothyronine. It has an essential regulatory function during vertebrate development. It has been found in brain, skin, uterus, placenta and fetal tissues. Although the roles of these enzymes in some physiological situations have been determined, their roles in others remain unclear. Reviewed in [St09, Peeters06, Kohrle02]. Human polymorphisms in the genes encoding the deiodinases have been described, although no inactivating mutations have been reported. Phenotypes of mice with targeted deletions of the deiodinase genes have been studied (reviewed in [Peeters06]). Although the three dimensional structures of these enzymes have not been determined, molecular models have been analyzed (reviewed in [Gereben08, St09]). Their homodimeric structure is critical for catalytic activity [Sagar08, CurcioMorelli03]. Rat type I deiodinase was reported to be microsomal [Toyoda95], but studies of kidney epithelial cells localized it to the basolateral plasma membrane [Leonard01] and in [Kohrle02]. Site-directed mutagenesis of recombinant enzyme has identified amino acid residues essential to catalysis and dimer assembly [Simpson06, Leonard05, Leonard01, Berry92]. Recombinant human enzyme encoded by gene DIO1 ([Toyoda95a, Mandel92]) has been expressed and assayed in a heterologous host [CurcioMorelli03, Kuiper02, Mandel92]. Gene Citations: [Kuiper05, Berry91, Berry91a] Locations: plasma membrane Map Position: [128,385,707 <- 128,404,014] Molecular Weight of Polypeptide: 29.73 kD (from nucleotide sequence) Enzymatic reaction of: 3',5'-diiodothyronine 5'-deiodinase (type I iododthyronine deiodinase) EC Number: 1.97.1.10 3',5'-diiodothyronine + a reduced electron acceptor <=> 3'-monoiodothyronine + an oxidized electron acceptor + iodide + H+ The reaction direction shown, that is, A + B ↔ C + D versus C + D ↔ A + B, is in accordance with the Enzyme Commission system. The reaction is favored in the direction shown. In Pathways: thyroid hormone metabolism I (via deiodination) Inhibitors (Competitive): 3,5,3'-triiodo-L-thyronine [Fekkes82] Kinetic Parameters: Substrate Km (μM) Citations 0.77 [Fekkes82] 3',5'-diiodothyronine Enzymatic reaction of: L-thyroxine 5-deiodinase (type I iododthyronine deiodinase) EC Number: 1.97.1.11 3,3',5'-triiodo-L-thyronine + iodide + an oxidized electron acceptor + H+ <=> L-thyroxine + a reduced electron acceptor The reaction direction shown, that is, A + B ↔ C + D versus C + D ↔ A + B, is in accordance with the Enzyme Commission system. The reaction is favored in the opposite direction. In Pathways: thyroid hormone metabolism I (via deiodination) Enzymatic reaction of: 3,3',5'-triiodo-L-thyronine 5'-deiodinase (type I iododthyronine deiodinase) EC Number: 1.97.1.10 3,3',5'-triiodo-L-thyronine + a reduced electron acceptor <=> 3,3'-diiodothyronine + iodide + an oxidized electron acceptor + H+ The reaction direction shown, that is, A + B ↔ C + D versus C + D ↔ A + B, is in accordance with the Enzyme Commission system. The reaction is favored in the direction shown. In Pathways: thyroid hormone metabolism I (via deiodination) Summary: The Km value is for wild-type, recombinant rat enzyme transiently expressed in human JEG-3 cells. Substitution of sulfur for selenium caused a 10-fold increase in Km for this substrate [Berry91a]. Cofactors or Prosthetic Groups: selenide [Behne90], L-selenocysteine [Berry91, Behne90] Kinetic Parameters: Substrate Km (μM) Citations 0.25 [Berry91a] 3,3',5'-triiodo-L-thyronine Enzymatic reaction of: L-thyroxine 5'-deiodinase (type I iododthyronine deiodinase) EC Number: 1.97.1.10 3,5,3'-triiodo-L-thyronine + iodide + an oxidized electron acceptor + H+ <=> L-thyroxine + a reduced electron acceptor The reaction direction shown, that is, A + B ↔ C + D versus C + D ↔ A + B, is in accordance with the Enzyme Commission system. The reaction is favored in the opposite direction. In Pathways: thyroid hormone metabolism I (via deiodination) , thyroid hormone metabolism II (via conjugation and/or degradation) Summary: This enzyme catalyzes the deiodination of L-thyroxine and its metabolites using a two-substrate ping-pong mechanism. In the first half-reaction the substrate interacts with a selenolate anion to form an enzyme-selenenyl-iodide intermediate containing an oxidized selenium atom. Reduction occurs in the second half-reaction using an unknown physiological reducing cosubstrate. Vicinal dithiols such as dithiothreitol are used as cosubstrate in vitro. Thiouracil inhibitors such as propylthiouracil inactivate the mammalian enzyme by reacting with the enzyme-selenenyl-iodide intermediate. propylthiouracil is used as a drug to treat severe hyperthyroidism (in [Berry91a] and in [Kohrle02]). In vitro, the enzyme also catalyzes 5-deiodination of L-thyroxine to 3,3',5'-triiodo-L-thyronine under alkaline pH conditions in the presence of dithiothreitol (in [Kohrle02] [Hoffken77]). In contrast, type II iododthyronine deiodinase has a sequential reaction mechanism and is not inhibited by propylthiouracil (in [Kohrle02]. Cofactors or Prosthetic Groups: selenide [Behne90], L-selenocysteine [Berry91, Behne90] Inhibitors (Irreversible): propylthiouracil [Kohrle02] Kinetic Parameters: Substrate Km (μM) Citations 2.0 [Koehrle86] L-thyroxine DEIODINASE, IODOTHYRONINE, TYPE I; DIO1 HYPERTHYROXINEMIA DUE TO DECREASED PERIPHERAL CONVERSION OF T4, INCLUDED TEXT Although thyroxine (tetraiodothyronine; T4) is the principal secretory product of the vertebrate thyroid, its essential metabolic and developmental effects are all mediated by triiodothyronine (T3), which is produced from the prohormone by 5-primedeiodination. The type I iodothyronine deiodinase, a thiol-requiring propylthiouracil-sensitive oxidoreductase, is found mainly in liver and kidney. Using expression cloning in the Xenopus oocyte, Berry et al. (1991) isolated a 2.1-kb cDNA for this deiodinase from a rat liver cDNA library. Its authenticity was confirmed by the kinetic properties of the protein expressed in transient assay systems, the tissue distribution of the mRNA, and its changes with thyroid status. Berry et al. (1991) found that the mRNA for iodothyronine deiodinase contains a UGA codon for selenocysteine which is necessary for maximal enzyme activity. The finding explains why conversion of T4 to T3 is impaired in experimental selenium deficiency and identifies an essential role for this trace element in thyroid hormone action. Previously the only enzyme known to contain a selenocysteine was glutathione peroxidase (138320). There is no apparent homology otherwise between the sequences of the 2 genes. Mandel et al. (1992) cloned a human iodothyronine deiodinase gene (designated 5-prime DI, or 5DI, by them) from liver and kidney cDNA libraries. The predicted protein has a molecular mass of 28.7 kD and contains a selenocysteine at position 382. The human gene is 88% similar to the rat homolog. The gene is also symbolized TXDI1 for thyroxine deiodinase type I. See also TXDI3 (601038) and TXDI2 (601413). Hatfield and Diamond (1993) pointed out that of all the genetic code words, UGA has played the largest number of distinct roles in evolution. In today's genetic language, UGA serves as a termination codon in the universal genetic code, a tryptophan codon in mitochondria and mycoplasma, and a selenocysteine codon in E. coli and in mammals. Indeed, UGA codes for selenocysteine in representatives of all the life kingdoms: monera, protist, plant, animal, and fungi. AUG has long been known to serve a dual role in the universal genetic code; it codes for the initiation of protein synthesis and, at internal positions of protein, By FISH, for Jakobs et al. (1997) mapped the methionine. human DIO1 gene to chromosome 1p33-p32. Jansen et al. (1982) described 2 patients, an 8-year-old boy and a 60-year-old woman, with elevated levels of serum thyroxine but normal serum triiodothyronine. The pituitary-thyroid axis could be normally stimulated by thyrotropin-releasing hormone. High levels of serum T4-binding globulin decreased during T3 treatment in the boy. In these patients, raised serum T4 was necessary to produce in the peripheral tissues sufficient T3 to maintain the euthyroid state. The authors suggested that the defect resides either in the transport of T4 into tissue cells or in 5-prime-deiodinase activity catalyzing the T4 to T3 conversion. Studies of the families showed no clue as to whether the disorder was hereditary. The boy was ascertained because of constitutional delay and problems in infancy related perhaps to toxemia of pregnancy and umbilical cord strangulation and amniotic fluid aspiration at birth. The woman had undergone subtotal thyroidectomy for Graves disease. Kleinhaus et al. (1988) described an 11-year-old girl with asymptomatic hyperthyroxinemia who remained euthyroid and healthy during 5 years of observation. Besides having elevated serum T4 concentrations, she showed low-normal or definitely low levels of deiodinated forms of T4. The girl had a small diffuse goiter, her serum TSH (see 188540) response to TRH was exaggerated, and thyroid radioiodine was elevated, suggesting slightly increased TSH secretion and, consequently, increased thyroid secretion. Kleinhaus et al. (1988) interpreted the findings as indicating reduced activity of several, and perhaps all, peripheral 5-prime-deiodination pathways, including possibly also thyrotroph T4 5-prime-deiodination. Thus, the girl appeared to have a previously unrecognized syndrome of generalized 5-prime-deiodinase deficiency. The genetic nature of the abnormality could not be determined; all relatives, including the parents and 4 sibs, had normal serum T4 levels and were healthy. Inbred mouse strains differ in their capacity to deiodinate iododioxin and iodothyronines, with strains segregating into high or low activity groups. Metabolism of iododioxin occurs via the type I iodothyronine 5-prime deiodinase. Berry et al. (1993) found that recombinant inbred strains derived from crosses between high and low activity strains exhibited segregation characteristic of a single allele difference. Linkage was performed using a restriction fragment length variant from the deiodinase gene. Linkage with previously mapped loci allowed assignment of the gene to mouse chromosome 4 in a region that shows extensive homology of synteny with the short arm of chromosome 1. Maia et al. (1995) identified an abnormality of the dio1 gene in mice with inherited deficiency of type 1 deiodinase. Toyoda et al. (1996) analyzed the exon/intron structure of the human DIO1 gene and compared it with that of a patient with suspected congenital type I deiodinase deficiency. The human gene is identical in exon/intron arrangement to the mouse gene, with coding sequences and a selenocysteine insertion sequence element contained in 4 exons. There were no mutations in the sequences of exons 1-4 of the patient's genomic DNA. Functional studies by transient expression techniques showed no difference in basal promoter activity or T3 responsiveness between the patient's and the normal gene. Thus, Toyoda et al. (1996) concluded that a structural abnormality in the type I iodothyronine deiodinase gene is not a likely explanation for this patient's deiodinase-deficient phenotype. Peeters et al. (2003) investigated the occurrence and possible effects of SNPs in the deiodinases (DIO1; DIO2, 601413; DIO3, 601038), the TSH receptor (TSHR; 603372), and the thyroid hormone receptor-beta (THRB; 190160) genes. They identified 8 SNPs of interest, 4 of which had not yet been published. Three are located in the 3-prime untranslated region: a C/T variation at nucleotide position 785 of the DIO1 cDNA, referred to as D1a-C/T (allele frequencies, C = 66%, T = 34%); an A/G variation at position 1814, referred to as D1b-A/G (A = 89.7= %, G = 10.3%); and a T/G polymorphism at nucleotide position 1546 of the DIO3 cDNA, referred to as D3-T/G (T = 85.5%, G = 14.2%). D1a-T was associated in a dose-dependent manner with a higher plasma reverse T3 (rT3), a higher plasma rT3/T4, and a lower T3/rT3 ratio. The D1b-G allele was associated with lower plasma rT3/T4 and with higher T3/rT3 ratios. The G allele of the TSHRc-C/G (asp727 to glu) polymorphism, TSHRc-G, was associated with a lower plasma TSH and with lower plasma TSH/free T4, TSH/T3, and TSH/T4 ratios. The authors concluded that they found significant associations of 3 SNPs in 2 genes (DIO1, TSHR) with plasma TSH or iodothyronine levels in a normal population. De Jong et al. (2007) studied the association of polymorphisms in the DIO1 (D1a-C/T, D1b-A/G) and DIO2 (D2-ORFa-Gly3Asp, D2-Thr92Ala) genes with circulating thyroid parameters and early neuroimaging markers of Alzheimer disease (AD; see 104300). Carriers of the D1a-T allele had higher serum free T4 and reverse rT3, lower T3, and lower T3/rT3. The D1b-G allele was associated with higher serum T3 and T3/rT3. They concluded that there is an association of D1a-C/T and D1b-A/G polymorphisms with iodothyronine levels in the elderly, and that polymorphisms in the DIO1 and DIO2 genes are not associated with early MRI markers of AD. Peeters et al. (2005) investigated whether genetic variations in DIO1 are associated with the insulin-like growth factor-1 (IGF1; 147440) system. In 156 blood donors and 350 elderly men, the association of DIO1 haplotype alleles with circulating IGF1 and free IGF1 levels was studied. In addition, they investigated potential associations with muscle strength and body composition in the elderly population. Finally the relation between serum iodothyronine levels and IGF1 levels was studied. In blood donors, haplotype allele 2 (D1a-T/D1b-A) was associated with higher levels of free IGF1. In elderly men, haplotype allele 2 also showed an allele dose increase in free IGF1 levels and an allele dose decrease in serum triiodothyronine (T3) levels, independent of age. In blood donors, tetraiodothyronine (T4) and free T4 were negatively correlated with total IGF1 levels, whereas T3/T4 and T3/reverse-T3 ratios were positively correlated with total IGF1. In conclusion, a polymorphism that results in a decreased DIO1 activity is associated with an increase in free IGF1 levels. The association of DIO1 haplotype allele 2 with serum T3 levels in the elderly population suggested a relative increase in its contribution to circulating T3 in old age. Role of D1 deiodinase in thyroid hormone activation and inactivation The functional role of D1 in humans remains a matter of debate. Nearly 80% of peripheral T 3 originates from deiodination of the pro-hormone T4 by D1 and D2. However, the relative roles of D1 versus D2 in extrathyroidal T 3 production are still controversial. Probably because D1 was the first selenodeiodinase to be identified and because of its subcellular location, it was often assumed that the majority of circulating T3 was derived from T4 via D1 activity. Nevertheless, the long-life of D1 protein and T3 upregulation of D1 activity, just the opposite of what would be expected in a typical feedback loop, raise some questions about this assumption. Moreover, clinical studies have showed a maximum decrease of 25% in serum T 3 in athyreotic, T4-replaced, euthyroid individuals treated with higher doses of PTU (Geffner et al. 1975, Saberi et al. 1975). Indeed, subsequent cumulative data on the biochemical and molecular properties of D2 indicate that this enzyme also contributes a significant portion of serum T3 levels in humans (Bianco et al. 2005, Maia et al. 2005). The D1 enzyme seems extremely inefficient in carrying out this reaction when compared with the D2, which has a 700-fold greater catalytic efficiency for 5′-deiodination of T4 (Maia et al. 2005). First, in contrast with D2-catalyzed reaction that generates 1 mol of T3 for each T4 molecule, for each 2 moles of T4 deiodinated by D1, only 1 mol of T3 and a second of rT3 is produced since D1 catalyzes the inner- and outer-ring deiodination of T4 equally well, as predicted from Vmax/Km estimates (Visser et al. 1988). This can explain a catalytic efficiency difference of 2, whereas the rest is primarily due to the much slower rate of D1-catalyzed T4 to T3 conversion under physiological conditions. This is not easily observed under typical in vitro assay conditions because the catalytic activity of this enzyme significantly varies from the artificial DTT generally used to other cofactor candidates such as GSH, as well as with variations of the cofactor concentrations (Goswami & Rosenberg 1987, Goemann et al. 2010). The human D1- or D2-derived T3 production significantly varies according to the thyroid status. Because DIO1 expression is positively regulated by thyroid hormone, D1-catalyzed T4 to T3 reaction is expected to be decreased in the hypothyroid state (Maia et al. 1995c, Kim et al. 1998). In contrast, increased D2-catalyzed T4 to T3 conversion is anticipated due to the increase in the half-life of D2 protein and DIO2 transcription secondary to the decreased levels of free T4 (FT4) and T3 (FT3) respectively (Gereben et al. 2000, Bianco et al. 2005, Wagner et al. 2007). In the opposite direction, D1-catalyzed T3 production would predominate in the hyperthyroid state (see below). These assumptions are consistent with the predicted increased efficiency of D2-catalyzed T4 to T3 conversion as demonstrated in early studies; these studies showed that in hypothyroid patients, the fractional whole-body conversion rate of T4 to T3 was 42%, whereas this rate fell to 21% in the same patients made euthyroid by l-T4 replacement therapy (Inada et al. 1975). Sustained elevations in serum T3/T4 ratios have also been reported in athyreotic subjects partially withdrawn from T4 therapy (Lum et al. 1984). In addition to the importance of the D1 and D2 in converting T4 to T3 in peripheral tissues, these enzymes are also highly expressed in the thyroid gland (Leonard & Visser 1986, Toyoda et al. 1992, Schoenmakers et al. 1995, Salvatore et al. 1996, Leonard et al. 2001). D1 seems to be constitutively expressed in the human thyroid gland, whereas D2 expression varies closely with the degree of thyroid stimulation (Salvatore et al. 1996). The thyroidal contribution for overall thyroid hormone economy is estimated on two-thirds from hydrolysis of thyroglobulin and one-third from deiodination (Laurberg et al. 2007). Interestingly, perfusion studies demonstrated that dog and human thyroid have a preferential secretion of T 3, favoring a more pronounced D1 activity in this gland (Laurberg 1978, Tegler et al. 1982). The distinct subcellular localization of D1 and D2 might also contribute to its functional roles. D1 is located in the inner surface of the plasma membrane, whereas D2 is in the endoplasmic reticulum (Baqui et al. 2000), which could explain the early observations that T3 generated by D1 rapidly equilibrates with plasma T3. Moreover, this deiodinase seems to be positioned much better to clear rT3 and other compounds from the circulation (Toyoda et al. 1997). The role of the facilitated deiodination of sulfated iodothyronines might be the recovery of the trace element iodine from inactive hormone metabolites, reutilized for T4 synthesis (Toyoda et al. 1997). On the other hand, D2-derived T3 production has a greater effect on T3-dependent gene transcription than that from D1, which indicates that generation of nuclear T 3 is an intrinsic property of the D2 protein (Maia et al. 2005). Important insights into the role of D1 for thyroid hormone metabolism were also obtained from two genetically modified animal models: C3H strains, which present inherited low D1 expression in liver and kidney, and D1KO mice, with targeted disruption of the Dio1 gene (Berry et al. 1993, Schoenmakers et al. 1993, Schneider et al. 2006). The reduced D1 activity in C3H mice correlates with a CGT repeat insertion into the 5′-flanking region of the Dio1 gene that seems to impair C3H promoter potency (Maia et al. 1995a). Both the strains have elevated serum total and FT4 and rT3 but normal serum-FT3 and TSH concentrations. The normal FT3 concentration in these mice could be explained by the fact that, although the fractional conversion of T 4 to T3 per day would be reduced, the higher FT4 concentration permits normal daily T3 production. In addition, serum T3 levels were significantly higher in C3H than in C57 mice after the same dose of exogenous T 3, suggesting a reduction in T3 clearance that might contribute to the maintenance of serum T3 concentrations (Maia et al. 1995c). However, the rate of disappearance of T3 was comparable in euthyroid WT and D1KO mice (Schneider et al. 2006). A potential source of serum T3 in both C3H and D1KO mice is D2-catalyzed T4 to T3 conversion. Indeed, it has been shown that, despite the higher serum-FT4, the levels of D2 activity in brown adipose tissue of C3H mice are comparable to those observed in C57 animals (Wagner et al. 2007). The normal serum-FT3 levels would lead to a euthyroid state in peripheral tissues and can account for the observed euthyroid phenotypes in both phenotypes. Of note, no cases of inherited D1 deficiency in human have been documented so far. In the last decade, animal models with deficiency of both D1 and D2 enzymes were generated (Christoffolete et al. 2007, Galton et al. 2009). The C3H-D2KO mouse (targeted disruption of the Dio2 gene and genetically low D1 expression) has hepatic and renal D1 activities lower than those observed in wild-type mice (C57/BL6) but unexpectedly higher than those of the C3H mice. These mice present euthyroid serum T3 levels and serum T4 levels even more augmented than in the C3H. The serum TSH is increased, which could be important to maintain euthyroid serum T 3 concentrations. The double D1/D2KO mice are also able to maintain a normal serum T3 level and their general health, growth, and reproductive capacity are seemingly unimpaired (Galton et al. 2009). The authors underline the feature that D1 and D2 enzymes might not be essential to the maintenance of normal plasmatic T3 levels in the rodent as long as the hypothalamic–pituitary–thyroid axis is intact. These alterations, however, might not perfectly reflect those putatively found in humans, because the rat thyroid is responsible for 50% of the circulating T3, whereas humans depend more on peripheral deiodination, given that only 20% of the T 3 is derived from the thyroid gland. Iodine is essential for thyroid hormone synthesis, and iodine deficiency leads a series of physiological adaptations in the hypothalamic–pituitary–thyroid axis in an attempt to maintain plasma and tissue T3 in the normal range. The earliest thyroidal modification is a decrease in 3,5-diiodotyronine, with a consequent decrease in the thyroidal T4, whereas thyroidal T3 remains constant (Riesco et al. 1976). Indeed, elevated plasma TSH, associated with decreased serum T 4 and a virtually unchanged T3, are the physiological hallmarks of moderate iodine deficiency as well as of the early phases of primary hypothyroidism. In both situations, however, the extrathyroidal changes are complex and involve a high degree of tissue specificity. It is observed that although D1 and D3 activity is unchanged in marginal iodine deficiency, D2 activity is markedly upregulated in D2-expressing tissues, thus increasing the proportion of T3 formed locally and mitigating the decreases in tissue T3 content (Larsen et al. 1981, Janssen et al. 1994, Schröder-van der Elst et al. 1998). Interestingly, however, increase in liver D1 activity has been observed in severe iodine-deficient rats under selenium supplementation (Arthur et al. 1991). Of note, studies in the D1KO mouse demonstrated that iodothyronines other than T4 are the substrates for 5′-deiodination by D1 enzyme, which releases iodine back into the circulation (Schneider et al. 2006). In the absence of D1, inactive and lesser iodothyronines escape deiodination and are excreted in the feces with the potential loss of the associated iodine. This scavenging function of D1 might be particularly important in the iodine deficiency setting (Galton et al. 2009). Recently, attention has been turned to a potential role of D1 in the biosynthesis of thyronamines (3-iodothyronamine, 3-T1AM, and thyronamine, T0AM), a newly identified class of endogenous compounds that seem to be isozyme-specific substrates of deiodinases (Scanlan et al. 2004, Piehl et al. 2008, Scanlan 2009). These molecules are chemical derivatives of thyronines, the principal chemical form of T4 that appears to antagonize the typical actions of thyroid hormones. It has been shown that the iodothyronamines have Vmax/Km values comparable to the corresponding iodothyronine, indicating that they would be as readily deiodinated as iodothyronines by the appropriate deiodinase isoenzyme (Piehl et al. 2008). Nevertheless, unlike T4, T4AM is not a substrate for D1 or D2 enzymes. Instead, T4AM is readily deiodinated to 3,3′,5′-triiodothyronamide (rT3AM) by D3, and rT3AM can be further deiodinated to ultimately provide T1AM. Using HepG2 cell lysates or mouse liver membrane fractions, D1 was able to use rT3AM or 3′,5′-diiodothyronamine (3′,5′-T2AM) as substrates and both reactions were sensitive to PTU. On the other hand, T1AM is the most efficiently processed thyronamine substrate using a sulfotransferase preparation from human liver, and the Vmax/KM value for T1AM compares to that of T3, suggesting that similar to T3, sulfation of T1AM may be an important clearance mechanism for regulating free circulating levels (Piehl et al. 2008). The physiological role of thyronamines is uncertain, but pharmacological data show that a single-dose T1AM administration in vivo induces intense hypothermia and bradycardia, leading to a hypometabolic state that may confer a potential neuroprotective benefit in cases of ischemic injury (Scanlan et al. 2004). Indeed, T1AM treatment was found to reduce infarct volume by 40% in a middle cerebral artery occlusion stroke model in mice. Interestingly, the degree of neuroprotection afforded by T 1AM was correlated with the magnitude of T1AM-induced hypothermia (Doyle et al. 2007). These findings indicate the existence of an exciting signaling pathway that leads to rapid physiological effects that are opposite to those produced by thyroid hormone excess (Scanlan 2009). Nevertheless, as pointed out by the authors, the precise relationship between T 1AM and thyroid hormone remains poorly understood, and there is currently no direct evidence for in vivo thyroid hormone-derived synthesis of thyronamines. Thyroid T4 5'-deiodinase activity in normal and abnormal human thyroid glands. To investigate the activity of the thyroid gland to convert T4 to T3, we measured the activity of thyroid T4 5'-deiodinase in the following human thyroid glands: 9 normal glands, 5 Hashimoto's thyroiditis, 13 follicular adenomas, 11 methimazole (MMI)treated Graves' disease (GD), 11 propranolol iodide-treated GD, and 8 propylthiouracil (PTU)-treated GD. The enzyme activity was determined by the ability of 100,000 X g pellet of the thyroid homogenate to convert T4 to T3 in vitro. Normal thyroids showed the enzyme activity of 1.59 +/- 0.18 (mean +/- SEM) pmol T3/mg protein/min. Euthyroid Hashimoto's thyroiditis displayed the enzyme activity of 1.01 +/- 0.15 pmol T3/mg protein/min, which was similar to the normal thyroid enzyme activity. The hypothyroid gland of Hashimoto's thyroiditis showed the enzyme activity of 1.8 pmol T3/mg protein/min. Follicular adenomas showed a wide range of enzyme activity with the mean level of 3.24 +/- 0.82 pmol T3/mg protein/min that did not differ significantly from that of the normal thyroids. Interestingly, one adenoma, despite TSH suppression that ordinarily decreases enzyme activity, showed the greatest activity of 11.0 pmol T3/mg protein/min. Graves' thyroids following treatment with MMI, PTU, and propranolol-iodide showed enzyme activities of 4.61 +/- 0.53, 3.95 +/- 0.43, and 3.51 +/- 0.46 pmol T3/mg protein/min, respectively; all these values were greater than that of the normal thyroids (P less than 0.01), but did not differ significantly when compared with each other. In summary, thyroid glands with Hashimoto's thyroiditis had activities of T4 to T3 conversion similar to the normal thyroid glands DEIODINASE, IODOTHYRONINE, TYPE II; DIO2 Description Type II iodothyronine deiodinase is a selenoprotein that catalyzes the 5-prime deiodination of thyroxine (T4) to generate an active thyroid hormone, 3,3-prime,5-triiodothyronine (T3) (Ohba et al., 2001). Cloning Croteau et al. (1996) identified and characterized rat and human DII cDNAs. Both code for selenoproteins and exhibit limited regions of homology with DI (DIO1; 147892) and DIII (DIO3; 601038). In the rat pituitary and brown adipose tissue, DII mRNA levels are altered more than 10-fold by changes in the thyroid hormone status of the animal. Northern analysis of RNA derived from human tissues revealed expression of DII transcripts in heart, skeletal muscle, placenta, fetal brain, and several regions of the adult brain. By EST database analysis, followed by 3-prime RACE of total thyroid RNA, Buettner et al. (1998) extended the DIO2 sequence isolated by Croteau et al. (1996) in the 3-prime direction and cloned full-length DIO2. The 3-prime sequence contains several AU-rich elements. Just upstream of the polyadenylation signal in the 3-prime UTR is a predicted stem loop structure with features of a form-2 selenocysteine insertion sequence (SECIS), which is required to decode a UGA codon as selenocysteine. Northern blot analysis of thyroid mRNA detected transcripts of about 6.5 and 7.5 kb. EST database analysis identified DIO2 clones from thyroid, brain, retina, placenta, breast, uterus, prostate, and skin libraries. In addition, DIO2 clones were found in several libraries derived from pooled tissues, such as fetal liver and spleen or fetal testis, B cell, and lung. Using Northern blot analysis, Bartha et al. (2000) detected DIO2 transcripts of about 6.8 and 7.5 kb in thyroid, pituitary, cardiac and skeletal muscle, and possibly brain, but only a 7.5-kb transcript was detected in placenta. PCR and endonuclease digestion indicated that there are 4 primary transcripts in thyroid: the full-length 7.5-kb transcript, a 7.2-kb transcript, and 2 shorter transcripts that use alternate start sites just upstream of the ATG start codon. By PCR, Ohba et al. (2001) identified 2 alternatively spliced DIO2 transcripts that include intronic sequences between the 2 invariant DIO2 exons. These splice variants showed tissue-specific expression. By SDS-PAGE of mesothelioma cell lysates, Curcio et al. (2001) determined that endogenous DIO2 has an apparent molecular mass of 31 kD. Immunolocalization of DIO2 in this cell line showed DIO2 costaining with an endoplasmic reticulum resident protein. Gene Function Croteau et al. (1996) noted that thyroid hormone appears to have important regulatory effects in some mammalian tissues, such as the developing brain, the anterior pituitary gland, and brown adipose tissue. A relatively high proportion of the receptorbound triiodothyronine is found within the tissue itself rather than in plasma. The expression in these tissues of type II iodothyronine deiodinase (symbolized DII by them), which catalyzes deiodination of thyroxine T4 exclusively on the outer ring (5-prime-position) to yield T3, suggests that DII is responsible for this 'local' production of T3 and is thus important in influencing thyroid hormone action in these tissues. In addition, DII activity is markedly elevated in the hypothyroid state and appears to be responsible for catalyzing the production of a large proportion of the circulating T3 under such conditions. Croteau et al. (1996) noted that, from the cDNAs of iodothyronine deiodinase types I and III, deiodinases are known to contain in-frame TGATGA codons that code for selenocysteine. The catalytic properties and tissue patterns of expression of these selenoproteins differ from those of DII. Unlike DII, DI is expressed in liver and kidney and is capable of inner ring deiodination of sulfated thyroid hormone conjugates. DIII functions as an inner ring deiodinase to convert T4 and T3 to inactive metabolites. Its expression in placenta and several fetal tissues during early development suggested that it plays a role in preventing premature exposure of developing tissues to adult levels of thyroid hormones. DII also is present in several fetal and neonatal tissues and is essential for providing the brain with appropriate levels of T3 during the critical period of development. Salvatore et al. (1996) reported that type 2 iodothyronine deiodinase (referred to as D2 by them) is highly expressed in human thyroid at levels 50- to 150-fold higher than in placenta. D2 mRNA was especially high in thyroids from Graves patients and in follicular adenomas. Stimulated thyroids had higher D2 to D1 (i.e., TXDI1) mRNA ratios than normal or multinodular glands, suggesting differential regulation of D1 and D2 expression. They concluded that intrathyroidal T4-to-T3 conversion by D2 may contribute significantly to the relative increase in thyroidal T3 production in patients with Graves disease, toxic adenomas, and, perhaps, iodine deficiency. Buettner et al. (1998) confirmed that the SECIS element in the 3-prime UTR of DIO2 has SECIS activity. A fragment containing the stem loop structure and the SECIS element hybridized to DIO2 mRNA in human thyroid. A G-to-A mutation in the essential AUGA motif in the SECIS element abolished SECIS activity. Transfection of the DIO2 coding region plus the 3-prime UTR in human embryonic kidney cells or injection of DIO2 cRNA in Xenopus oocytes resulted in expression of DIO2 with deiodinase activity. The distance between the SECIS element and the UGA codon affected DIO2 activity. Bartha et al. (2000) identified a canonical cAMP response element (CRE) in the DIO2 promoter region that drove cAMPdependent expression of a reporter gene. Primary human thyroid cell cultures increased basal expression of DIO2 in response to forskolin, confirming the cAMP responsiveness of the endogenous DIO2 gene. Curcio et al. (2001) found that selenium depletion reduced the basal endogenous DIO2 activity in a mesothelioma cell line. This depletion could be reversed by selenium supplementation in a dose- and time-dependent fashion. DIO2 activity also increased following exposure to a nonhydrolyzable cAMP analog. Exposure to the thyroxine substrate increased the degradation of DIO2, resulting in decreased DIO2 activity. The short half-life of endogenous DIO2 (less than 1 hr) and the increased degradation of DIO2 in the presence of thyroxine were reduced or eliminated by exposure to proteasome inhibitors. Thyroid hormone signaling during a postnatal period in the mouse is essential for cochlear development and the subsequent onset of hearing. To study the control of this temporal dependency, Campos-Barros et al. (2000) investigated the role of iodothyronine deiodinases, which in target tissues convert the prohormone thyroxine into T3, the active ligand for the thyroid hormone receptor (see 190120). They found that D2 activity rose dramatically in the mouse cochlea to peak around postnatal day 7 (P7), after which activity declined by P10. This activity peaked a few days before the onset of hearing, suggesting a role for D2 in amplifying local T3 levels at a critical stage of cochlear development. A mouse cochlear D2 cDNA was isolated and shown to have near identity to rat D2. In situ hybridization localized D2 mRNA in periosteal connective tissue in the modiolus, the cochlear outer capsule, and the septal divisions between the turns of the cochlea. D2 expression in these regions that give rise to the bony labyrinth was complementary to thyroid hormone receptor expression in the sensory epithelium. Thus, the connective tissue may control deiodination of thyroxine and release of T3 to confer a paracrine-like control of thyroid hormone receptor activation. These results suggested that temporal and spatial control of ligand availability conferred by D2 provides an important level of regulation of the thyroid hormone receptor pathways required for cochlear maturation. DIO2 mRNA is abundant in the human thyroid but very low in adult rat thyroid, whereas DIO1 activity is high in both. To understand the molecular regulation of these genes in thyroid cells, Gereben et al. (2001) studied the effect of TITF1 (NKX2-1; 600635) and PAX8 (167415) on the transcriptional activity of the deiodinase promoters. Both the approximately 6.5-kb human DIO2 sequence and its most 3-prime 633 bp were activated 10-fold by transiently expressed TITF1 in COS-7 cells, but human DIO1 was unaffected. Surprisingly, the response of the rat DIO2 gene to TITF1 was only 3-fold despite the 73% identity with the proximal 633-bp region of human DIO2, including complete conservation of a functional cAMP response element at -90. Neither human nor rat DIO2 nor human DIO1 was induced by PAX8. Two sites in human DIO2, both of which are absent in rat DIO2, have significant affinity for, and are required for the full response to, TITF1. Curcio-Morelli et al. (2003) stated that D2 activity is terminated by its ubiquitination and proteasome-mediated degradation. By yeast 2-hybrid analysis, they found that the deubiquitinating enzyme VDU1 (USP33; 615146) bound the C terminus of D2. Coimmunoprecipitation analysis in transfected HEK293 cells showed that both VDU1 and VDU2 (USP20; 615143) bound D2. Both VDU enzymes colocalized with D2 in the ER and reduced ubiquitination and degradation of D2. Expression of Vdu1, but not Vdu2, increased in mouse brown adipocytes following exposure to cold or norepinephrine. Curcio-Morelli et al. (2003) concluded that VDU enzymes recycle inactive ubiquitinated D2 to its active deubiquitinated form and thereby regulate the supply of active thyroid hormone. Watanabe et al. (2006) showed that the administration of bile acids to mice increased energy expenditure in brown adipose tissue, preventing obesity and resistance to insulin. This novel metabolic effect of bile acids is critically dependent on the induction of the cAMP-dependent thyroid hormone activating enzyme type 2 iodothyronine deiodinase (D2), shown by the loss of this effect in D2-null mice. Treatment of brown adipocytes and human skeletal myocytes with bile acids increased D2 activity and oxygen consumption. These effects are independent of FXR-alpha (see 603826), and instead are mediated by increased cAMP production that stems from the binding of bile acids with the G protein-coupled receptor TGR5 (610147). In both rodents and humans, the most thermogenically important tissues are specifically targeted by this mechanism since they coexpress D2 and TGR5. Watanabe et al. (2006) concluded that the bile acid-TGR5-cAMP-D2 signaling pathway is therefore a crucial mechanism for fine-tuning energy homeostasis that can be targeted to improve metabolic control. Gene Structure Celi et al. (1998) determined that the coding region of the DIO2 gene covers 8.1 kb and contains 2 exons separated by a long intron. Bartha et al. (2000) determined that the 5-prime UTR of the DIO2 gene contains a functional CRE. It also has an AP1 (165160) site, several TATA or TATA-like sequences, and 3 transcriptional start sites. Bartha et al. (2000) identified a DIO2 splice variant that uses intronic sequences in addition to the 2 exons described by Celi et al. (1998). Ohba et al. (2001) determined that the long intronic sequence of the DIO2 gene contains 2 alternatively spliced sequences used by some DIO2 splice variants. Mapping By radiation hybrid analysis, Celi et al. (1998) mapped the DIO2 gene to chromosome 14q24.3. Using FISH, Araki et al. (1999) mapped the DIO2 gene to chromosome 14q24.2-q24.3. Animal Model DIO2 is a selenoenzyme that catalyzes the conversion of T4 to T3 via 5-prime-deiodination. It is expressed in the pituitary, brain, brown adipose tissue, and the reproductive tract. To examine the physiologic role of DIO2, Schneider et al. (2001) developed a mouse strain lacking Dio2 activity. Mice homologous for the targeted deletion (Dio2 knockout mice) had no gross phenotypic abnormalities, and development and reproductive function appeared normal, except for mild growth retardation (9%) in males. Serum T4 and TSH levels were both elevated significantly (40% and 100%, respectively) in the Dio2 knockout mice, suggesting that the pituitary gland is resistant to the feedback effect of plasma T4. This was supported by finding that serum TSH levels in hypothyroid wildtype mice were suppressed by administration of either T4 or T3, but only T3 was effective in the Dio2 mouse. Ng et al. (2004) found that D2-deficient mice had defective auditory function, retarded differentiation of the cochlear inner sulcus and sensory epithelium, and deformity of the tectorial membrane. They concluded that the similarity of the D2-deficient phenotype to that caused by deletion of thyroid hormone receptor genes suggests that D2 is essential for hearing and that D2 confers on the cochlea the ability to stimulate its own T3 response at a critical developmental period. Type II iodothyronine deiodinase is an enzyme belongs to the iodothyronine deiodinase family. It activates thyroid hormone by converting the prohormone thyroxine (T4) by outer ring deiodination (ORD) to bioactive 3,3',5-triiodothyronine (T3). It is highly expressed in the thyroid, and may contribute significantly to the relative increase in thyroidal T3 production in patients with Graves disease and thyroid adenomas. This protein contains selenocysteine (Sec) residues encoded by the UGA codon, which normally signals translation termination. The 3' UTR of Sec-containing genes have a common stem-loop structure, the sec insertion sequence (SECIS), which is necessary for the recognition of UGA as a Sec codon rather than as a stop signal. Alternative splicing results in multiple transcript variants encoding different isoforms. DEIODINASE, IODOTHYRONINE, TYPE III; DIO3 Cloning Thyroid hormone is critical to the normal development of the human central nervous system. Salvatore et al. (1995) noted that, despite the presence of thyroxine (T4) and thyroid follicles in the fetal thyroid by 10 to 12 weeks of gestation, as well as the potential availability of maternal thyroid hormone, the free concentration of the active thyroid hormone T3 is less than half that of maternal levels up to the time of delivery. The physiologic rationale for this circumstance is not well understood, but the authors suggested that it is possible that 'normal' circulating T3 concentrations could have deleterious effects on immature tissues or could enhance the metabolic requirements of the fetus. There are 2 principal mechanisms by which the circulating fetal T3 concentration is maintained at low levels. One is that the type I iodothyronine deiodinase (147892) in fetal liver is expressed at lower levels relative to those in adult life. This reduces the extra thyroidal T3 supply from this source. The second important factor in maintaining low serum T3 concentrations is the expression of high levels of the type III deiodinase in placenta of all species examined. Type III iodothyronine deiodinase catalyzes the conversion of T4 and T3 to inactive metabolites. Salvatore et al. (1995) cloned human placental type III iodothyronine deiodinase (which they referred to as D3). It is a selenoenzyme, as evidenced by (1) the presence of an in-frame UGA codon at position 144; (2) the synthesis of a 32-kD (75)Se-labeled protein in D3 cDNA transfected cells; and (3) the presence of a selenocysteine insertion sequence element in the 3-prime untranslated region of an mRNA that is required for its expression. The authors stated that the D3 selenocysteine insertion sequence element is more potent than that found in the type I deiodinase or glutathione peroxidase (138320) gene, suggesting a high priority for selenocysteine incorporation into this enzyme. The conservation of this enzyme from Xenopus laevis tadpoles to humans implies an essential role for regulation of thyroid hormone inactivation during embryologic development. By Northern blot analysis, Hernandez et al. (2004) detected several DIO3 transcripts. A 2.1-kb transcript was highly expressed in placenta, fetal liver, and uterus, and a 3.2-kb transcript predominated in testis, bladder, and uterus. A 4.8-kb transcript was detected in heart and skeletal muscle, but it hybridized only with the most 5-prime region of the DIO3 cDNA, suggesting that it is not a true coding transcript. Some or all of these transcripts were also present in adrenal cortex, thyroid, prostate, stomach, pancreas, and fetal lung. Gene Function Huang et al. (2000) reported the case of a 3-month-old infant with massive hepatic hemangiomas and primary hypothyroidism who needed very high doses of thyroid hormone to restore euthyroidism and normal thyrotropin secretion. This finding suggested that the rate of degradation of thyroid hormone was accelerated. They subsequently identified high levels of type III iodothyronine deiodinase activity in the hemangioma tissue. Normally present in the brain and placenta, this selenoenzyme catalyzes the conversion of thyroxine to reverse triiodothyronine and the conversion of triiodothyronine to 3,3-primediiodothyronine, both of which are biologically inactive. They then retrospectively analyzed other patients with hemangiomas and identified additional patients with similar histories and other hemangiomas with type III iodothyronine deiodinase activity. Gene Structure Hernandez et al. (2004) determined that, like the mouse Dio3 gene, the coding region and 3-prime UTR of human DIO3 are contained within a single exon. In both mouse and human, the promoter elements are located immediately upstream and are extremely GC rich (80% of the sequence). Mapping By FISH, Hernandez et al. (1998) mapped the human DIO3 gene to 14q32 and the mouse Dio3 gene to 12F1. See also DIO1 (147892) and DIO2 (601413). Hernandez et al. (2004) noted that mouse Dio3 is imprinted and preferentially expressed from the paternal allele during fetal development. They determined that exon 1 of the DIO3OS gene (608523), which is transcribed in the opposite orientation of the DIO3 gene, maps to a region 1 kb upstream of the DIO3 transcription start site and within the DIO3 GC-rich promoter region. Animal Model By targeted inactivation of the Dio3 gene in mouse embryonic stem cells, Hernandez et al. (2006) generated Dio3-knockout mice, which demonstrated neonatal thyrotoxicosis followed later by persistent central hypothyroidism. Early in life, the mutant mice had delayed T3 clearance, markedly elevated serum T3 levels, and overexpression of T3-inducible genes in the brain. From postnatal day 15 to adulthood, Dio3-knockout mice exhibited central hypothyroidism, with low serum levels of T4 and T3, and modest or no increase in TSH (see 118850) concentration; peripheral tissues were also hypothyroid. Hypothalamic T3 was decreased, whereas thyrotropin-releasing hormone (TRH; 613879) expression was elevated. Hernandez et al. (2006) concluded DIO3 plays a critical role in the maturation and function of the thyroid axis. Type 3 Deiodinase and Consumptive Hypothyroidism: A Common Mechanism for a Rare Disease Abstract The major product secreted by the thyroid is thyroxine (T4), whereas most of the biologically active triiodothyronine (T3) derives from the peripheral conversion of T4 into T3. The deiodinase enzymes are involved in activation and inactivation of thyroid hormones (THs). Type 1 and type 2 deiodinase (D1 and D2) convert T4 into T3 whereas D3 degrades T4 and T3 into inactive metabolites and is thus the major physiological TH inactivator. The hypothalamic-pituitary-thyroid axis maintains circulating TH levels constant, while the deiodinases tissue-specifically regulate intracellular thyroid status by controlling TH action in a precise spatio-temporal fashion. Here we review the data related to the recent identification of a paraneoplastic syndrome called “consumptive hypothyroidism,” which exemplifies how deiodinases alter substantially the concentration of TH in blood. This syndrome results from the aberrant uncontrolled expression of D3 that can induce a severe form of hypothyroidism by inactivating T4 and T3 in defined tumor tissue. This rare TH insufficiency generally affects patients in the first years of life, and has distinct features in terms of diagnosis, treatment, and prognosis with respect to other forms of hypothyroidism. Introduction Thyroid hormones (THs) are circulating hormones widely involved in the development and metabolic homeostasis of virtually all mammalian tissues. Alterations in serum TH levels in the fetus and newborns cause significant deficits in experimental animals and in humans (1). The importance of maintaining the TH-dependent transcriptional signature range is supported by the presence of diverse homeostasis checkpoints. In fact, beyond the hypothalamus-pituitary-thyroid axis, which responds to changes in serum levels of thyroid-stimulating hormone (TSH) (2), there are various ways to affect thyroid status in a specific tissue. First, TH exerts its genomic action only in tissues that express a thyroid hormone receptor (TR), and second, it is possible to locally modulate the amount of TH available for cells at a pre-receptoral level. TH transporters and deiodinases are important in determining the availability of TH in a tissue-specific manner. Although THs are lipophilic molecules, they require specific plasma membrane transporters to enter target cells. Various transporter families have been identified, but only monocarboxylate transporters 8 and 10 (MCT8 and MCT10) and anion transporting polypeptide 1C1 (OATP1C1) have elevated specificity for THs. These proteins are differentially expressed in several tissues: OATP1C1 is expressed in liver, kidney, and brain, MCT8 is highly expressed in liver and brain, and MCT10 is expressed in intestine, kidney, liver, and placenta. A further level of complexity results from the capacity of these transporters to determine the efflux of THs from the intracellular environment to the external milieu (3). The clinical relevance of these transporters is supported by evidence that mutations in the gene encoding MCT8 are associated with elevated serum triiodothyronine (T3) levels and severe sex-linked psychomotor retardation (Allan–Herndon–Dudley syndrome) (4). Once inside the nucleus, T3 acts through the binding to ligand-dependent transcription factors namely TRs, which bind, mainly as heterodimers with retinoid-X receptors, to TH response elements in target genes (5). TRs are encoded by two genes, THRA and THRB, which are located on chromosomes 17 and 3, respectively. TRα has one T3-binding splice product, TRα1, predominantly expressed in brain, heart, and skeletal muscle, and two non-T3-binding splice products, TRα2 and TRα3, and several additional truncated forms. TRβ has three major T3-binding splice products: TRβ1 is almost ubiquitously expressed; TRβ2 is expressed primarily in the brain, pituitary, retina, and inner ear; and TRβ3 is expressed in kidney, liver, and lung (6). Selenodeiodinases catalyze the activation (D1 and D2) and inactivation (D3) of the THs thyroxine (T4) and 3,5,3′triiodothyronine (T3) by removing distinct iodine moieties. D1 and D2 convert thyroxine into the most active metabolic form of TH, T3, by outer ring deiodination; and D3 converts T4 and T3 into the inactive forms rT3 and 3,3′-diiodiothyronine (T2), respectively, by inner ring deiodination. D1 is expressed mostly in the liver, kidney, thyroid, and pituitary; D2 is expressed primarily in the thyroid, central nervous system, pituitary, developing cochlea, brown adipose tissue, and skeletal muscle; D3 is prevalently expressed in many fetal tissues, through the adult it is expressed in placenta, brain, and skin, and to a lesser extent in the pregnant uterus and pancreatic B-cells. The three deiodinases exert different actions: D1 participates in T3 production within the thyroid gland and controls circulating T3 levels, whereas D2 and D3 are active in local deiodination processes. Recent evidence indicates that D1 and D2 are the major sources of circulating T3 in euthyroid humans, with D1 being the major source of circulating T3 in hyperthyroid patients (7). Conversely, D3 inactivates T3 at tissue and plasma level (8). A dramatic example of the potency of the D3 pathways in TH clearance, at both systemic and tissue level, is the newly identified consumptive hypothyroidism syndrome, which is a rare condition resulting from increased D3 production in neoplastic cells and consequent T4 and T3 catabolism. In this review, we focus on D3 as the cause of consumptive hypothyroidism, a newly recognized clinical entity which often requires close cooperation between clinical endocrinologists, pediatricians, and oncologists. Type 3 Deiodinase and Inactivation of Thyroid Hormones As mentioned, type 3 deiodinase is the physiological inactivator of TH: it catalyzes deiodination on the inner rings of T3 and T4 to produce T2 and rT3, respectively. D3 is a selenoenzyme encoded by the DIO3 gene in humans; it is localized on chromosome 14q32 (9). The DIO3 genomic structure contains a single exon and, at the 3′-UTR, a specific RNA structure, named SECIS (selenocysteine insertion element), that is crucial for the insertion of the selenocysteine residue and for maximal enzymatic catalytic efficiency (10). The DIO3 gene is imprinted, with preferential expression of the paternal allele. It belongs to a cluster of imprinted regions, at the DLK1-DIO3 locus (11). D3 is an integral membrane protein that exerts its role as a homodimer (12). It is recycled through a system of endosomal clathrin-coated vesicles. This suggests a possible mechanism for D3 reactivation, and furthermore the possibility that this enzyme acts on both extracellular and intracellular pools of T3 and T4 (13). Diverse signals are able to regulate D3 expression in vitro and in vivo: retinoic acid, serum growth factors, estrogens and progesterone, TGFβ, Wnt-βcatenin, and Shh/Gli2 increase D3 levels, whereas glucocorticoid and growth hormone reduce D3 levels (14, 15). Recent data from our laboratory implicates p63, a member of the p53 family (16), in D3 regulation (unpublished data). D3 plays the essential role of protecting tissue from excessive TH levels under normal and disease conditions. Indeed, thyrotoxicosis of any cause induces D3, whereas hypothyroidism suppresses D3 (17). D3 activity is elevated during development, a time when circulating fetal TH levels are much lower than those of the mother (18). D3 is widely expressed in such embryonic tissues as liver, cerebral cortex, cardiovascular apparatus, gonads, gut, skin, and urinary tract (19). The human placenta also expresses elevated D3 levels. Within the placenta, D3 blocks the maternal-to-fetal transfer of T4 thereby protecting the fetus from TH excess (20, 21). During late neonatal and adult life, D3 expression is restricted to a few tissues. It has been detected in skin, the central nervous system, and some endocrine glands (22). However, in adult life, D3 expression is reactivated in several disease conditions, namely inflammation, liver regeneration, cardiac hypertrophy and infarct, and cancer (15, 23–25). Several studies have demonstrated that thyroid status affects tumor formation, growth, and metastasis, which suggests that THs are involved in cell transformation. D3 expression has been widely documented in a variety of malignancies; it is turned-on in some malignant cell lines (26) and in a number of human tumors, i.e., oligodendromas, astrocytomas, gliosarcomas, glioblastoma multiforme, TSH-secreting pituitary adenomas, basal cell carcinomas, colon adenomas, and carcinomas (25). It has been postulated that D3 activity is required to facilitate tumor cell proliferation (27). Consumptive Hypothyroidism The paraneoplastic syndrome consumptive hypothyroidism is a rare form of hypothyroidism first identified in newborns with infantile hepatic hemangiomatosis (HHE) (28). The latter is a benign tumor of vascular origin affecting 4–5% of white infants. In most cases, the natural course of HHE is characterized by a rapid proliferation phase during the first 1–2 years of life, and by a slow and steady decline over the next 5–7 years until complete spontaneous involution of the tumor mass. The spectrum of vascular lesions that could be termed “HHE” ranges from benign and self-limiting to aggressive and life-threatening neoplasias. About 10% of cases have aggressive characteristics based on their size, location, and the number of lesions (29). In 2000, Huang et al. reported the first case of severe hypothyroidism (defined as “consumptive hypothyroidism”) in a 6-week-old infant with HHE (28). At the time of diagnosis, TSH level was elevated (156 mUI/ml) and the serum free thyroxine (FT4) concentration was low. Hence, the infant was treated with prednisolone (2 mg/kg) for the hemangioma, and levothyroxine (LT4) replacement (37.5 μg/day) for hypothyroidism, but 16 days later the TSH value was still 256 mUI/ml. At the age of 3 months, the child had intermittent bradycardia and hypothermia, which were probably linked to the hypothyroid status as evidenced by thyroid function tests. In fact, TSH concentration was 177 mUI/ml, serum T4 concentration was 2.5 μg/dl, T3 concentration was low (15 ng/ml), and the level of rT3 was elevated (413 ng/ml). Based on these TH values, replacement hormone therapy was increased to 50 μg/day of LT4, and intravenous administration of 90 μg/day liothyronine was initiated. This therapy resulted in normalization of TSH and T3, but T4 levels remained low. This replacement dose of hormone therapy is very high considering that an athyreotic infant of the same age requires about 7–10 μg/kg/day of LT4 (equivalent to about 3 μg/kg/day of T3), which is about nine times lower than that required by this patient. After a vertical midline abdominal fasciotomy and embolization of the multiple hemangiomas, the patient died from systemic complications. At that time, the cause of hypothyroidism was not clear, but a markedly elevated serum thyroglobulin concentration argued against a diagnosis of congenital hypothyroidism. Furthermore, biochemical TH parameters suggested that THs were normally synthesized and secreted, but that their rate of degradation was increased. The overexpression of D3 and its activity in the hemangioma tissue were 0.78 pmol of T3 deiodinated/min/mg of protein, a value 7.5 times higher than that normally present in placental tissue, the highest physiological D3-expressing tissue in humans (20). To date, more than 20 cases of consumptive hypothyroidism secondary to neoplasias have been reported (Table (Table1).1). An evaluation of all these cases strongly suggests that the development of consumptive hypothyroidism is related to the elevated D3 enzyme, which is in turn directly proportional to the size of the tumor mass and its specific activity, and independent of its localization. Furthermore, most cases described so far support the hypothesis advanced by Huang that D3 activity increases rapidly during the proliferative phase of hemangioma (28). It is also well recognized that consumptive hypothyroidism develops when the catabolic D3 activity exceeds the physiological or exogenous replacement of THs (Figure (Figure1).1). The direct relationship between consumptive hypothyroidism, tumor size, and D3 activity is demonstrated by the spontaneous remission of hypothyroidism and normalization of serum rT3 concentration upon the natural regression of HHE or after surgical resection of the tumor (Table (Table11). Table 1 Summary of published cases of “consumptive hypothyroidism.” Figure 1 Schematic illustration of the pathogenesis of condition named “consumptive hypothyroidism.” Recent studies have shed light on the role of D3 in the control of cell proliferation in tumors. In basal cell carcinoma the constitutive activation of the Shh-Gli pathway directly induces D3 mRNA, which in turn reduces TH intracellular activity thereby resulting in increased cyclin D1 expression and keratinocyte proliferation (15). In colon cancer, D3 is a direct target of β-catenin and its activation is a key factor in the regulation of cell proliferation in this tumor (25). Taken together, these findings suggest that local attenuation of TH is an important step in the primary proliferation of some neoplastic cells, which might explain the sustained D3 levels particularly in the initial phase of hemangioma growth. What induces D3 in hemangioma cells? Data from in vitro studies suggest that D3 overexpression in hemangioma is induced by basic fibroblast growth factor and vascular endothelial growth factor. These angiogenic factors play an important role in the pathogenesis of hemangioma. Indeed, recent evidence suggests that propranolol, an antagonist of β2 adrenergic receptors, is able to block tumor growth by inhibiting the expression of these factors in endothelial tumor cells (30). In most cases, HHE responds to treatment with steroids and/or propanol. If this first-line therapeutic strategy fails, surgery may be necessary, i.e., tumor resection, liver transplantation, or ligation of the hepatic artery (29). The treatment of HHE-associated hypothyroidism can problematic because exogenous hormones are massively converted to inactive forms. The aim of treatment in such cases is to normalize the T4 level, which is critically important particularly for the developing brain during the neonatal period. Hypothyroidism may be very severe, hence large doses of TH are necessary to normalize the T4 level. Doses of LT4 should be increased gradually until TH levels normalize. Since T4 is rapidly converted to the inactive rT3 form, combined therapy with liothyronine may be necessary. Moreover, in severe cases of HHE, parenteral LT4 administration, with or without liothyronine, may be used to bypass the liver and the hemangioma filter (31). Hypothyroidism usually resolves with involution of the tumor, and LT4 treatment may be gradually reduced as involution progresses (32, 33). Therefore, thyroid functions must be frequently monitored to ensure maintenance of the euthyroid status. Monitoring at 6-week intervals is not sufficient for a rapidly developing condition like consumptive hypothyroidism, and weekly monitoring of T4 and T3 levels should be considered particularly in the initial phase of treatment. Consumptive hypothyroidism is diagnosed based on the detection of D3 activity in the tumor tissue of a patient with biochemical and clinical signs of hypothyroidism. However, HHE biopsy may be a risky procedure due to the high vascularity of the tumor. Hence, consumptive hypothyroidism should be suspected in each HHE patient whose TH values rapidly change especially during the proliferative phase. Usually, the increased D3 activity is mirrored by an elevation of rT3 level associated with a supraphysiological requirement for exogenous hormone and an high serum thyroglobulin level. In such patients, hypothyroidism usually resolves after medical or surgical treatment of HHE. The differential diagnosis is between congenital or acquired hypothyroidism, TSH-secreting pituitary adenoma and a hormone resistant state. However, a rapid increase of TSH, low levels of T3 and elevated levels of rT3 associated with rapid proliferation of a vascular tumor is typical of consumptive hypothyroidism. Elevated serum thyroglobulin levels, a normal or increased thyroid uptake or the presence of thyroid gland verified by ultrasound can exclude a diagnosis of congenital hypothyroidism (34). Very rarely, a TSH-like factor may be secreted by HHE, as reported in one infant with hypothyroidism and HHE (35). In such cases, the FT4 index is normal in congenital hypothyroidism and elevated in TSH-secreting pituitary adenomas. In conclusion, given the long-term negative effects of TH alterations on brain and on overall development, thyroid functions must be frequently monitored in patients with HHE. Conversely, since consumptive hypothyroidism has also been described in two infants with asymptomatic HHE (36, 37), HHE should be suspected in all infants with severe unexplained hypothyroidism. Consumptive hypothyroidism has been described also in adults. The first reported case was a 21-year-old girl with a large hepatic HHE and hypothyroidism manifested with thyroid enlargement, a serum TSH concentration of 26.2 mU/L and normal serum free T4 in the absence of thyroid antibodies (38). Despite the normal FT4 and total T3 levels, serum rT3 was fivefold higher than normal without significant illness or liver failure. The hypothyroidism and goiter resolved after liver transplantation. Subsequently, Ruppe et al. (39) reported the first case of consumptive hypothyroidism associated with a “non-vascular tumor” in an adult who required an elevated dose of LT4 before resection of a large malignant fibrous tumor. Recently, Howard et al. described a 38-year-old athyreotic woman who presented elevated levels of TSH (37.3 mU/L) after the discovery of a large HHE. The patient also had low T3 and elevated rT3 serum levels (40). An interesting aspect of this case was an increase in TSH after partial hepatic resection despite hormone therapy. This may have been due to the hepatic resection itself. Indeed, regenerative processes in hepatic tissue are associated with rapid induction of D3 in post-hepatectomized mice, so one may speculate that hepatic D3 reactivation occurs in humans after liver resection, and this, in turn, causes an increase in TSH levels (24). Other Conditions Associated with Elevated D3 Levels Consumptive hypothyroidism has also been associated with extra-hepatic HHE. In fact, two cases of consumptive hypothyroidism associated with cutaneous hemangioma have been reported in infants (40). Moreover, a case of neonatal hemangioma in the skin, associated with consumptive hypothyroidism, has been reported in an 8-month-old infant (46). The first case of consumptive hypothyroidism secondary to a large parotid hemangioma was reported in 2012 in a child with congenital hypothyroidism and hypoplastic thyroid gland (47). The first case of consumptive hypothyroidism from a nonvascular tumor was reported in 2005 in a patient with a large malignant solitary fibrous tumor, expressing elevated D3 mRNA, and proteins (39). At diagnosis, the patient was administered a supraphysiologic dose of levothyroxine prior to the discovery of the tumor, whose identification suggested that the cause of hypothyroidism could have been the paraneoplastic syndrome. Notably, D3 is frequently overexpressed in malignant cells, which raises the question “why are circulating TH levels not always perturbed by neoplastic cells?” It is likely that, in most conditions, due to the low D3 expression in tumor cells, and more importantly, to the efficient homeostatic hypothalamic-pituitary-thyroid axis, paraneoplastic D3 is unable to perturb significantly the circulating level of TH. Finally, tyrosine kinase inhibitors have been recently proposed as therapeutic options for patients with recurrent non-resectable radio-resistant thyroid cancer. In some of these patients undergoing LT4 replacement therapy, treatment with these drugs has been associated with an increase in TSH level. This increase has been associated with the induction (probably at hepatic level) of D3 and, the consequent need to increase the amount of T4 in replacement therapy (48). Conclusion In conclusion, increased catabolism of TH by increased levels of D3 can cause a rare form of acquired hypothyroidism. While this rare syndrome was initially described in association with massive hemangiomas in infants, it was later recognized also in adults, and, in some cases, as a consequence of non-vascular tumors. Although this condition is usually reversible, it requires prompt recognition and immediate TH replacement therapy usually at higher doses than required in other types of hypothyroidism. Furthermore, given that D3 reactivation in tumors is a rather common event in the neoplastic context in vitro, and that D3 increase may be a side effect of new pharmacological treatments, it is conceivable that consumptive hypothyroidism may be more frequent than expected. For the clinician, this form of hypothyroidism requires particular attention given its refractory nature toward replacement therapy and the risk of mental and growth retardation in neonatal patients.