Tanaka_Kado - Center for Science, Technology, Medicine
advertisement

Scientific Analysis of Radiation
Contamination at the Area around the
Fukushima-Daiichi Nuclear Power
Station
Saturo Tanaka and Shinchiro Kado, School of Engineering, The University of
Tokyo
Author correspondence information:
NONE PROVIDED
Paper submitted to the Nuclear Engineering & Society working paper series
Abstract
The purpose of this article is to introduce some background knowledge to analyze
the release of radioactive materials from the Fukushima Dai-Ichi (1F) nuclear power
station (NPS) based on their inventory in the reactor core, mechanisms of the
release and the behavior of the released radionuclide from the scientific points of
view. Basic knowledge on contamination of the area and decontamination are also
described.
Introduction
At the Fukushima Dai-Ichi (1F) nuclear
power site, six nuclear power plants are
located. Among them, units 1, 2, and 3
(1F1, 1F2 and 1F3 respectively, caused big
accidents by earthquake and tsunami on
Mar. 11, 2011. The NPSs - 1F1, - 1F2 and 1F3 encountered the station blackout
(SBO; loss of all A/C power including
emergency diesel generator), back-up
battery depletion, and emergency cooling
system failure, resulting in a core melt in
the reactor pressure vessels (RPVs) at 1F1,
2 and 3, rupture of the suppression
chamber (suspected) at 1F2, and hydrogen
explosion in the reactor buildings 1F1 and
1F3. Image of these damages and the
pathways of the radioactive materials are
shown schematically in Fig. 1. These
events, together with the leakage of the
primary containment vessels (PCVs),
caused significant release of the
radioactive nuclides to the environment.
Figure 1 Schematic drawing of the reactor
damage and behavior of radioactive materials
In this article we made trials to evaluate the release of radioactive materials from
the NPS and their environmental behaviors based on the chemical and physical
properties of the radioactive materials and based on the data analysis of the
radiation dose rate.1
Methods of analysis
General ideas of the model
Figure 2 shows schematically the behavior of radioactive materials in the
environment after its release from the reactor facility. In order to evaluate the
radiation dose to the people we must know inventory of radionuclides and chemical
ele-ments in fuel just before the accident, release from the fuel at the accident,
existence states of radionuclides in RPV, PCV and reactor building, release from the
Note that the data evaluated here have considerable ambiguities, so authors would like to
suggest readers to take them as examples for the study. Indeed, up to now, the given data
from the government, TEPCO etc. has frequently updated.
1
2
stack or reactor building, migration in the atmosphere, contamination of soil and
radiation dose from radionuclide in the soil and in the atmosphere.
There are
basically two
approaches to
evaluate the
amount of
environmental
release of the
radionuclides.
One is based on
the analysis of
the physical
and chemical
conditions of
the core fuel. In Figure 2 Radioactive materials in the environment
this approach,
the fraction of the released amount would be approximated. The other is based on
the monitoring of the radiation dose increased due to the release from the plant and
deposition to the land.
Evaluation of the inventory
Amounts of radioactive nuclides, such as fission products (FPs), U, Pu and minor
actinides (MAs) in the reactor fuel need to be evaluated. Information about the
chemical elements is also important in the stoichiometric estimation of the chemical
forms of released fission products. It can be calculated with the help of the ORIGEN
code [1], which is based on the theory of production and the following radioactive
decay of the FPs and MAs.
Release from the fuel
A cause for release of radioactive materials at all reactors was that decay heat of
fission products had not been eliminated due to loss of the cooling function.
Consequently the fuel rods were exposed to the steam and the fuel and cladding
were heated up, which resulted in generating hydrogen gas by chemical reaction
between zirco¬nium and the steam above about 900 ˚C. The reaction Zr+2H2O =>
ZrO2+2H2 produces hydrogen which caused subsequent hydrogen explosions. It
also produces heat because this reaction is exothermic. This heat accelerates the
heating of the fuel combined with decay heat. At high temperature uranium made
eutectic compound with zirconium. Melting point of this eutectic is lower than uranium oxide. Figure 3 shows high temperature phenomena of the fuel. Some
radioactive materials in the fuel to be soluble in UO2 were released following
heating and melting of the fuel. A fraction of released radioactive materials from the
3
heated fuel depends on the vapor pressure (i.e., melting point) and diffusivity in the
fuel. These behaviors are strongly temperature dependent. A release rate constant k
[min-1] as a function of the temperature T [K] is given by
k k0 exp(Q / RT)
(1)
where Q is the activation energy [kcal/mol], and R = 0.001987 kcal/mol K the
universal gas constant.
Figure 3 High temperature phenomena in the core
Although Q depends on the chemical species,
ORNL and others proposed, in their CORSOR-O
model [2], to use the common Q of 55 kcal/mol
for all species and the dependence on the
species is represented by the empirically
corrected k0. For example, k0 =12,000 min-1
for Cs and Kr while 9,600 min-1(=0.8 x k0 (Cs))
for I and Te. The results are shown in Fig. 4.
Using CORSOR-O model, the fraction of
inventory released from the fuel at time t is
given by . Taking Cs as an example of
calculation, the fractions of inventory released
at 1,800 ˚C are: F = 90 % at t = 2 hours; and F =
100 % at t = 4 hours.
Figure 4 Temperature of release rate
constants from UO2 fuel
4
Severe accident progression analysis codes
In order to analyze or predict the severe accident progression, many computer
codes have been developed. MAAP (Modular Accident Analysis Program) was
developed by U.S. industries while MELCOR has been developed by U.S.NRC (United
States Nuclear Regulatory Commission) [3].
These codes basically calculate the thermal response of the core, dealing with whole
progression from the initiating event to the radionuclide releases to the
environment, which is called "source term". Therefore, initial inventory and the
release properties for each nuclide are required as input parameters. These values
are usually calculated based, for exam-ple, on the ORIGEN or CORSOR. The whole
progression from the initial event includes damages in RPV and PCV and consequent
leakage of water and steam.
Atmospheric transport model
Behavior of the radioactive materials released from a NPS differs for their chemical
properties and/or the weather con-ditions (e.g., wind direction, wind speed, rainfall,
snowfall) during the accident and the geography around the plant. No-ble gases
such as Kr or Xe are transported and dispersed by wind. If upward wind is
predominant, the gases will be transported to the stratosphere and delivered to the
entire earth by the wind. Gases of volatile radioactive materials such as I2 are also
transported by the wind. CsI or Cs oxides can be transported by the wind if these
nuclides float in the air as dust particles or attach to the aerosols. This is called the
"plume" as schematically shown in Fig. 2.
If rain or snow falls, some particles will fall out to the surface of the earth together
with the raindrops (wash-out or rain-out) and contaminate the land. Therefore,
prediction of the transportation of the radionuclide plume is crucial from the
viewpoint of radiation protection.
Note, on the other hand, that relatively large/heavy particles such as fuel grains
cannot transport far by the wind, so that they tends to fall out by gravity near the
NPS.
The time-integrated concentration of the released nuclides in the atmosphere,
χ(x,y,z) [Bq/h], can be predicted based on the Gaussian model developed by
Pasquill's as :
(x, y,z)
y2
z2
exp( 2 )exp( 2 )
2U yz
2 y
2 z
,
(2)
where Γ is the release rate at source [Bq/s], U the mean wind speed in the x
direction [m/s] [4]. The diffusion parameters, σy and σz, as a function of x and the
air-stability (having 6 categories, A-E), represent the broadening in transverse and
vertical direction, respectively. They are larger than that deduced from molecular
collisional diffusion, since the turbulent flows enhance the net diffusion.
5
SPEEDI (System for Prediction of Environmental Emergency Dose Information) is
the computer code in which the atmospheric transport of the specific particles can
be predicted using meteorological conditions and topography based on the basic
diffusion equation above. SPEEDI predict the behavior of released radioactive
materials for regional area using an atmospheric dynamic equations model with
meteorological data and topography and for local area using a mass-consistent wind
field model. Improved system (WSPEEDI) can predict in detail the process of the
atmospheric dispersion of released radioactive materials by turbulence and wet
deposition by rainfall.
The behavior of radioactive materials released to the ocean is evaluated from
transportation and dispersion along the ocean current, dispersion by the tidal
stream and wind, precipitation to the bottom of the sea and intake by fishes and
their migration. The compartment model is used for evaluation of the contamination
in the ocean.
Occurrence of the accident
and release, transport and
washout of the radiation
plume
Fig.5 shows a temporal evolution of
radiation dose rate observed inside 1F
site, nearby and distant cities together
with the wind conditions at 1F
monitoring posts (MPs). The direction
and the speed of the wind have been
recorded in 16 point compass, e.g. N,
NS, N, S, NWN.... , at the same time
with the radiation dose rate at the
monitoring posts/car in 1F. In order
to compare the temporal evolution of
the wind vector with other events, we
represented the wind direction θ as
the sine and cosine components of the
direction (orienting to the east be 0°,
while orienting to the north be 90°).
In the present analysis, sin(θ) > 0
Figure 5 Temporal evolution of the radiation dose rate6
at (a) 1F monitoring post, (c) nearby place & (d) far
place. (b) wind direction indicated by sine & cosine
components. [Data other than in the Univ. Tokyo were
mainly from the public release by TEPCO & MEXT].
corresponds to the direction from the land to the ocean, while cos(θ) < 0
corresponds to the direction to south (towards Tokyo).
From the severe-accident analysis
based on MAAP or MELCOR code, it is
reported that the core damage incident
for each unit happened approximately
at the period listed in Table 1.
In 1F site, the radiation dose began to
increase at 5:10 on Mar. 12 (14.5 hours
after scram), which coincides with the
incidents in 1F1 --- A. Vent and the
following hydrogen explosion may be
the cause of the increase in the
radiation dose in Minami-soma, 26 km
north from 1F, on Mar. 12 --- B.
Simulation Analysis
MAAP Code (TEPCO)
Unit
1F1
Core Exposure
3h
Core Damage
4h
RPV melt15 h
through
MELCOR Code (NISA)
Unit
1F1
Core Exposure
2h
Core Damage
3h
RPV melt5h
through
Actual Events
IC/ RCIC stopped 2h50
Vent (AO bulb)
23h44
Explosion/Ruptu 24h50
re
1F2
75 h
77 h
109 h
1F3
40 h
42 h
66 h
1F2
75 h
77 h
80 h
1F3
41 h
44 h
79 h
70h39
?
77 h
35h56
42h30
68h12
The core damage incident in 1F3
occurred on Mar.13. The venting of the
Table 1 Hours from scram (2011-Mar-11 14:46 JST)
PCV of 1F3 was operated several times
to depressurize the RPV during Mar.13 and 14, and hydrogen explosion also
occurred at 11:01 on Mar. 14 (68 hours after scram). Fortunately, the wind directed
to the East (sea direction) during the period --- C.
The incident at 1F2 caused the most serious release of the radioactive nuclides. The
leakage of the PCV (suspected) caused the release of the radioactive gasses around
21:30 on Mar. 14, which was several hours before the detection of the sound of
explosion or rupture at the suppression chamber (SC) of 1F2 (at 6:10) ---D. Note
that at this moment, we have no enough evidence to tell whether the event was the
explosion or the rupture -- on Oct. 2, 2011, it has been reported that the accidents
investigation commission in Tokyo Electric Power COmpany (TEPCO) regarded
from the signals recorded on a quake mater that the hydrogen explosion might not
occur.
7
The radioactive leakage from 1F2
could initiate the radiation plume
toward the south direction, and the
increase of the radiation dose was
observed as the plume propagated
and passed through the locations
at the speed of about 10 km/h-Fig.5, mark E. The radiation plume
was observed even in Tokyo (SW
230 km south from 1F) or Sizuoka
(SW 360 km). Fig. 6 shows the
temporal evolution of the radiation
dose observed at different
locations after initiating the
prominent radioactive release on
Mar. 15.
[ North < 50 km from 1F ]
The plume on Mar. 15 was soon
passed away and the radiation
dose decreased rapidly in
particular in far places. How-ever,
plume initiated by the SC rupture
propagated to the Northwest
direction and causes a
fallout/washout/rainout due to the
rainfalls and/or snowfalls. It
contributed the significant
increase in dose rate in these areas
such as Iitate-mura (NW 40 km). --- F
Figure 6 Temporal evolution of radiation dose rate of far
places. Right axis corresponds to rain. Note: data for office
0.5 km far from 1F divided by 10 to scale its temporal
behavior consistent with that of main gate (GateM) and
west gate (GateW).
After Mar. 16, release of
radioactive material was
decreased but still continued. As a result, rainfalls over the wide area in the South
direction washed out the plume into the soils, leading to a significant increase in the
radiation dose. This time the decrease in the dose rate was dominated by the
radiation decay of the radioactive nuclides.
On Mar. 18 and 19, wind directed to north direction and several peaks of the dose
rate were observed in Minami-soma(N 30 km). However, presumably because there
was no rainfall, these plumes did not deposit onto the ground. ---G
On Mar. 21, although rain felled in Fukushima, plume did not deposit to Minamisoma, because wind directed to South. ---- H
8
It suggested that the ground contamination occurred by both the plume and
rainfalls.
[South 50 ~ 100 km from 1F]
Ibaraki prefecture, located South of Fukushima, underwent considerable degree of
washout/rainout on 16 and 20, that can be found from the increase of the baseline
of the radiation dose, having the decay timescale of 131I, 8.02d. --- I
Just after the delivery of the plume, the decay of the short-lifetime radioactive
nucleus was also observed, such as 135I (6.7h), or 132I in radiative equilibrium with
132Te (78h) -- J
[South > 100 km from 1F]
In Tokyo, the rain on Mar. 21 washed out the plume and increase in the radiation
dose, which led to a kind of small panic when 131I was detected from the source of
tap water. ---- K
In Shizuoka, at 360 km from 1F, one can see from the time difference of the rain and
the increase in the dose rate, plume arrived during the rainy weather. ---L
It suggests that the plume stay no longer than a few days.
This speculation agrees with the observation of radioactive material level of fallout
in Tokyo per day [5]. Usually the fallout last around 3-4 days in March and April.
Namely, for the purpose of self-protection from the radioactive exposure, it is
preferable to watch the dose rate near one's location and the rain for a few days
after passing through the plume. Especially, the cesium deposition onto soils is
caused by the rain, while removal of the deposit cesium is difficult. Therefore, the
covering of the playground with plas-tic sheet before rain might have been effective
as an emergency protection of the ground contamination -- even a mattress/blanket
is better than nothing. Some prefecture office and the nuclear power plant provide
the real-time dose rate. It might be preferable if one could watch these data together
with the rain and wind speed in the weather forecast. At the same time, it might be
required that not only the government but also the scientists provide appropriate
information about how to interpret the monitored data.
Evaluations
Approach based on the radionuclide release analysis
Behavior of released radioactive nuclides is complicated because it is closely related
to the evolution of the accident. However, we roughly evaluated it assuming that a
9
certain proportion of the inventory in the fuel existing one day after the scram was
released.
Figure 7 is an illustrative image
of behavior of radioactive
materials in the reactor and
release to the environment. Environment to the release is
basically composed of two
steps: release from fuel (A) and
release to the environment
after release from the fuel (B).
The latter release mechanism
is complicated because detailed
information of reactor damage
and RI behavior in the
damaged reactor is not simple. Figure 7 Release fraction of leagage from reactor
Radionuclides exist in various
chemical forms in RPV, PCV and reactor building such as gas, dissolved in water,
aerosol, solid particle. Entrainment of the species to the gas phase is also important
in the dynamic or boiling state.
The electric output powers, the
number of fuel assemblies and
the average burn-up at the
scram of Unit 1(1F1), 2(1F2)
and 3(1F3) of the 1F-NPS are
(460 MWe, 400, 26 GWd/t),
(784 MWe, 548, 23 GWd/t) and
(784 MWe, 548, 22 GWd/t),
Figure 8 The inventories of radionuclide at 1F1 at one day
respectively. Using these data, after scram
amounts of radionuclides and
chemical elements for FP and MA at one day after the scram were calculated using
ORIGEN code. Figures 8 and 9 show the inventories of radionuclide and chemical
elements in 1F1 at one day after the scram, respectively. Inventories in 1F2 and 1F3
at one day after the scram are about 1.5 times of 1F1. The following nuclides were
found to be important from the produced amount and half-life: 239Np (2.4d), 133Xe
(5.25d), 140La (40.3h), 141Ce (32.5d), 131I (8.04d), 137Cs (30.17y), 134Cs (2.06y) 89Sr
(50.0d), 90Sr (29.1y) 132Te (78.2h), 129mTe (33.6d), 238Pu (87.7y), 239Pu (24,000y),
241Pu (14.4y), 140Ba (12.7d), 95Zr (64d), 91Y (58.5d), 127Sb (3.9d), 99Mo (66.0h), 3H
(12.3y) and 85Kr (10.7y). Chemical inventory shown in Fig. 9 gives us important
information. For example the inventory of Cs is about ten times larger than that of I.
10
The chemical state can typically be categorized into noble gases (Kr, Xe), volatile
materials (I, Cs, Te, H) and low vola-tile materials (Sr, Y, Pu). The degree of
volatilization is a key to understand the release at the accident.
The chemical forms and their location of
radioactive materials released from the
fuel depend on their chemical properties. Noble gases such as Kr and Xe exist
in the gas phase and are released to the
atmosphere by venting operation.
Iodine was released as CsI and dissolved Figure 9 The inventories of chemical elements in
in the water. However, some exist in the 1F1 at one day after the scram
gas phase as I attached to aerosol, I2
and or-ganic iodine. Cs takes the chemical forms of CsOH and oxide as well as CsI in
the gas phases or the water. Te exists as oxide in the gas phase or is dissolved in the
water. Sr is dissolved in the water as cation or exists as oxide in the gas phase or the
water. Therefore aerosols in the gas phase might carry these kinds of species.
In order to evaluate radionuclide release from fuel, we assumed the two cases of the
temperature and the duration time as (i) 2,800˚C and one hour, and (ii) 2,000 ˚C and
four hours. We used source terms based on the inventory at one hour after scram
for 1F1, 1F2 and 1F3 for simplicity. The fraction of inventory released from the fuel
is calculated by the release rate constants in as shown for these two cases in Table.
2., As can be seen from the comparison, all noble gasses and volatile materials are
released for both cases. However, the difference in the fraction is remarkable in Ba,
Sr, La and Pu.
2800˚C 1hour
Xe, Kr
100%
H
100%
Cs
100%
I
100%
Te
100%
Ba
83%
Sr
58%
Zr
1.7%
Np
1%
Mo
100%
La
1.7%
Pu
0.17%
Am
1%
2000˚C 4hour
Xe, Kr
100%
H
100%
Cs
100%
I
100%
Te
100%
Ba
29%
Sr
16%
Zr
0.34%
Np
0.5%
Mo
99%
La
0.34%
Pu
0.034%
Am
0.5%
The chemical properties such as vapor
pressure of each element must be
taken into account on the release rate
from RPV and PCV as shown in Fig. 7.
Some appropriate assumptions must
be taken for estimate of released
amount by the accident except noble
gases (fully released). Released
radioactive materials exist in the water
or the steam of RVP and PCV due to
their chemical properties and damages
of RPV and PCV. Particles in the fuel
generated by rapid cooling after
Table 2 The fraction of inventory released from the fuel
melting might be dispersed in the
water. Some parts of the radionuclide
in the gas phase are released in the accident. Some part of the species in the liquid
11
phase is considered to be transported to the gas phase by the entrainment. Release
fraction to the environment from the reactor is very complicated because it reflects
various events causing RI release such as seat leakage from the flanges or bulbs
(including SRV release to a leaking vessel), venting, hydrogen explosion, damage in
the suppression chamber. Therefore we tentatively assumed the release fraction
((B) in Fig. 7) from the RPV+PCV+Reactor Building to atmosphere considering vapor
pressure of the elements: Xe 100%; Kr 100%; Cs 1 %; I 1%; Te 0.1%; Sr 10-4; Ba 104; Zr 10-4; Np 10-4; Pu 10-5; 3H 25 % etc. In this evaluation we also assumed these
releases occurred at one day after the scram by the earthquake. Although these
assumptions are different from the actual accident scheme, our estimation can give
us fundamental understanding of RI release,
Amounts released to the environment can be calculated by "inventory" time
"release fraction from the fuel ((A) in Fig. 7)" times "release fraction from the
reactor ((B) in Fig. 7)". The calculation results of amounts released are: 3H 9.4 x 1014
Bq; 85Kr 7.6 x 1016 Bq; 89Sr 3.9 x 1014 Bq; 90Sr 3.5 x 1013 Bq; 129mTe 2.9 x 1014 Bq; 131I
6.0 x 1016 Bq ; 133Xe 1.3 x 1019 Bq; 137Cs 7.6 x 1015 Bq; 134Cs 7.4 x 1015 Bq; 249Np 8.8 x
1013 Bq; 241Pu 1.4 x 1010 Bq; 241Am 8.9 x 107 Bq for fuel damage at 2800 ºC for one
hour. The 131I equivalent amount of radionuclide is evaluated as 4.9 x 1017 Bq using
the conversion factor by INES manual.
NISA (Nuclear and Industrial Safety Agency) reported it as 7.7 x 1017 Bq calculated
by MELCOR code [6].
As to the release points of radioactive materials, these are released from the
venting stack in not severely damaged accident. However, in 1F accident, these were
also released from the disrupted points of PCV, duct pipes and reactor building.
Moreover contaminated water was released into the sea through the tunnel of 1F2
from a crack in a concrete pit.
Approach based on the radiation monitor
Result of the standard method based on the SPEEDI simulation
NSC (Nuclear Safety Commission of Japan) reported the source term of 131I and
137Cs released between Mar. 12 and Apr. 5 based on the atmospheric dispersion
simulation such as SPEEDI or WSPEEDI. The simulation result for the unit release
rate (1 Bq h-1) was compared with that obtained with the dust-sampler to normalize
the absolute value. They obtained: 150 PBq for 131I, and 12 PBq for 137Cs (1 PBq =
1015 Bq ). In order to obtain the radiological equivalence to 131I release, the value for
137Cs was multiplied by 40, yielding the total 131I equivalent release of 630 PBq [6]. --- These data was made minor correction to 570 PBq (131I: 130 PBq and 137Cs: 11
PBq) on Aug. 22.
However, the results of the SPEEDI calculation have only disclosed one on Mar. 23,
another one on Apr. 11, then finally the government admit that more than 5000
12
evaluation results had exist from the beginning of the accident and had not be
opened being afraid of the panic.
Because these evaluations rely on the simulation code and detailed weather data,
that is inaccessible by the public, we proposed a simple but straightforward
estimation from the radiation dose data, or radiation map, available at many
locations.
Alternative method based on the ground shine
The total release of the radioactive material, integral of the source term with
respect to the period of release, can be roughly evaluated from the ground
contamination after the plume passed through and being fallout/rainout/washout,
based on the following equations.
1
Dj (tobs ) Aj (tcom )
2
D̂ j (t obs ) D j (t obs ) /
tobs tcom / j
CF gnd, j
D (t
j
obs
)
(3)
(4)
j
1
Aj (ts ) D̂j (tobs )
2
Si Aj (ts )
(ts tobs )/ j
1
1
CFgnd,
j SF
Land Ocean
Land
(5)
(6)
where D and A represent the dose rate [Sv/h] and the radio activity of the surface
area [Bq/m2], respectively.
CFgnd is the conversion factor
from the ground contamination
to the dose rate at 1m high above
the ground, [(Sv/h)/(Bq/m2)]
shown in Fig. 10, while SF is the
shielding factor depending on the
ground condition or location or
buildings. We examined that
SF=0.7 is a plausible value to be
Figure 10 Schematic drawing of evaluation of dose rate
based on the ground shine.
applied in the present
situation(see Appendix B). τ is
the half life of the radioactivity. tcom, tobs and ts are the time when the species ratio is
determined, when the dose rate was measured, and when the radioactive species
are released, respectively. Note that the subscript j is the label of the species and 131I
(τ = 8.02 d), 134Cs (τ = 752.4 d) and 137Cs (τ = 11019.3 d) in the present case.
The ratio of the radioactivity A' can be determined at the specific time when all
species of interest can be commonly determined from the measurement such as
dust sampling or the soil analysis or from the simulations.
13
We adopted the Becquerel ratio
[131I]:[134Cs]:[137Cs] = 1:1:1 on tcom=
Apr. 10 from the air sampling data at
the Comprehensive Nuclear-Test-Ban
Treaty(CTBT) National Operation
System of Japan in Takasaki Gunma
prefecture [7].
In this case, taking tobs = Apr. 5 for
instance, the normalized dose ratio
can be calculated to be 0.21: 0.57:
0.22 from the Eq(1) followed by the
Figure 11 Dose rates at the location inside 20 km no-go
normalization using Eq (2). This ratio zone, scaled to that on Mar. 15 when dominant
radioactive release occurred.
agrees with the species-sensitive
dose monitoring in Ichihara-city,
Chiba prefecture reported by Japan Chemical Analysis Center [8].
About Eq (3), we corrected data from the dose rate the places of which were
categorized into the following several groups.
(i) Inside 20 km no-go zone:
For inside the 20 km no-go zone, TEPCO monitored the dose rate in Mar.30-Apr. 2
and Apr.18-19, the result of which are listed with the distance from 1F [9]. The data
is shown in Fig. 11 as a function of the distance from the 1F site. Although the
scattering of the data was significant having directional dependence, the general
trend exhibited decaying property. Therefore, we performed an exponential decay
fitting to determine the rough integral of the dose rate in this area based on the
following equation,
D j (0)
r
4 r exp( )dr 4 D j (0)L2
L
(7)
0
where, L is the characteristic length of the spatial decay while Dj(0) is the dose rate
extrapolated to the origin, namely 1F plant.
(ii) East part of Fukushima prefecture outside the 20 km no-go zone, and
(iii) West part of Fukushima prefecture:
The authority of Fukushima prefecture conducted a gamma dose rate monitoring at
more than 1,600 schools all over Fukushima except inside the 20 km circle from 1F
in Apr. 4-7, 2011 [10].
In this period, the contribution of 131I to the dose rate was still obvious, so can be
used to evaluate the radioactive release.
14
A dose rate map of
these data is shown in
Fig. 122 together with
the 1-dimentioal
distribution projected
along the latitude
direction.
It may safely be said
that the Fukushima
prefecture outside the
20 km no-go zone was
divided into two parts,
East and West areas of
which are
approximately equal.
We distributed the
1370 points to (ii) and
267 points to (iii)
depending on the
location. As a result,
one data point can be
Figure 12 Dose rate mapping of schools in Fukushima performed in April.
regarded as being
representing around
4.57 and 28.6 km2, respectively (area of the half disk 20 km in radius was removed
from the half area of the prefecture in (ii)).
(iv) Ibaraki prefecture:
Ibaraki prefecture, located South of Fukushima prefecture exhibits relatively higher
dose rate among the adjacent prefecture. We assumed the excess dose rate of the
Mito-city on Apr. 10, 0.17 µSv/h as being representing the averaged values of
Ibaragi prefecture.
(v) Tochigi, Chiba, Gunma prefectures and others:
For the minor correction of the evaluation the area of these three prefectures were
regarded as representing the ground contamination in the distant places. The excess
dose rate was assumed to be 0.07 µSv/h on Apr. 10.
The largest ambiguity in evaluating the released radioactive materials is in the
fraction of the ground deposition against the total release. Atmospheric simulations
also have considerable amount of error depending on the modeling.
Figure 12 is the projection along the latitude. Fukushima map from "National Land
numerical information (Administrative Divisions, 2011), Ministry of Land, Infrastructure,
Transport and Tourism, file: japan_ver71, processed by esri Japan"
2
15
I-131
Cs-134
Cs-137
We had first approximated the fraction
(i)
8.03
0.95
0.93
was about half. Actually, ref.[11]
(ii)
12.5
1.47
1.44
reported from the atmospheric
(iii)
2.33
0.27
0.27
dispersion model GEARN in WSPEEDI-II
(iv)
1.35
0.16
0.16
that these fractions for 131I and 137Cs are
(v)
1.63
0.19
0.19
0.44 and 0.46, respectively - i.e. can be
Total
25.8
3.05
2.98
suspected that the fraction for 134Cs is
land
0.13/0.44 0.22/0.4 0.22/0.46
also 0.46. However, ref [12], on the other fractio
6
hand, implies, from a three‐dimensional
n
Source 199/59
13.8/6.6 13.6/6.5
chemical transport model, Models‐3
I-eq.
199/59
38.8/18 542/258
Community Multi-scale Air Quality
Table 3 Evaluated ground contamination and
131
134
(CMAQ), that the fractions for I, Cs source terms in PBq
and 137Cs deposit on land were 0.13,
0.22 and 0.22, respectively. We have
updated the evaluation using these value range. The results were shown in the 3rd
raw in Table 3. Smaller values are the result adopting ref [12] as the fraction for the
land deposition, while larger values are those adopting ref. [11].
From these results, 131I equivalent
released radioactive nucleus was
evaluated to be 336-780 PBq.
The databases necessary to evaluate
the ground contamination can be
compiled in Appendix B. Note that the
ambiguity in 137Cs has a large effect on
the 131I equivalent value, since the
multiplying factor is as large as 40.
In this rough evaluation, different
from NSC, we did not care about the
daily change of the release rate and
based on the assumption that almost
all released species had been fallout/rain-out/wash-out and the
fraction of those on the land has been
contributing to the dose rate.
Ambiguity in the fraction that was
deposited on land also has a direct
effect on the evaluation of the source
terms. One needs to remind that it is
also true for the other method based
on the atmospheric transport
simulation.
Figure 13 Map of deposition of radioactive cesium
(sum of Cs-134 and Cs-137) for the land area within
80 km of the Fukushima Daiichi plant, reported by
MEXT.
16
Crosscheck of the evaluation:
The result in the previous subsection was compared to the cesium radiation map of
June 14 over Fukushima and adjacent prefectures and was presented by MEXT on
Sep.30. 1732 points are located in Fukushima Prefecture[13,14].
By correcting the data to that on Mar. 15, using Eq.(1), total ground contamination
scaled on Mar. 15 of Fukushima prefecture of 134Cs and 137Cs are 2.5 and 2.6 PBq,
yielding source term of 32 and 470 PBq, respectively, which agree well with the sum
of our evaluation (i), (ii) and (iii).
Fall-out of 131I, 134Cs and 137Cs has been monitored at specific cities in every
prefectures in Japan except Fukushima and Miyagi[15] .We summed up the monthly
fall-out in Bq/m2 in each prefecture multiplied by the area of each prefecture in m2.,
yielding 0.4 PBq for 137Cs. Value roughly agree with our evaluation for the some of
(iv) and (v).
Moreover, we would like to emphasize that these different methods lead to
consistent results with each other for source terms, release rates, dust sampling,
fall-outs, and dose rate by ground shine with SF=0.7 (Appendix B).
Fig. 13
Comparison between approaches
Evaluation results based on these approaches are compared in Table 4. All of them
exceeded the criteria of INES accident level 7 (>1016 Bq) . For the comparison the
results for the Chernobyl accident is also listed. One may understand that the rough
evaluations, nearly hand calculations, could obtain approximated release amount of
radioactive materials.
Method
Radionucli
de release
analysis
§ 4.1
Radiation
monitor
§ 4.2.2
NISA
NSC
ORIGEN
CORSOR-O
Chemical
analysis
MELCOR
Radiation map
(Ground shine)
SPEEDI
Dust Sampling
I-131
equivalent
I-131
x1
Cs-134
x 2.8
Cs-137
x 40
490
60
7.4
7.6
370
(770)
130
-----
6.1
336-780
59-199
6.6-13.8
6.5-13.6
630(570)
150(130)
-----
12(11)
Chernobyl
Core inventory
5200
1800
----85
analysis code
Table 4 Comparison between different approaches to evaluate the total release in PBq. Some data have
been updated from the initial publication, indicated in the round brackets.
17
It is worthwhile to mention that the total amounts of radionuclide of Chernobyl
accident was 1800 PBq for 131I, and 85 PBq for 137Cs, yielding the radiological
equivalence to 131I of 5200 PBq. It is considerably larger than that of FukushimaDaiichi accident. Reason for this fact if not only because the PCV covers the RPV in
the present case, but also because in the Chernobyl case, the massive amount of Cs, I,
Sr and Pu was released to the environment by steam explosion of the melted fuel.
Contamination and clean up the environment
The air dose, surface dose and
radioactivity in soil have been
measured around the site after
the accident. The detailed
distribution maps of radiation
dose are made with smaller
mesh than as before. Fig. 13
presents that massive amounts
of radiation fell to the surface of
the ground by snowfall after the Figure 14 Becquerel Ratio of Sr90/Cs137 [%] as a function of
the distance from 1F. Color of dots corresponds to the
plume, which contained
latitude, but no clear tendency has been observed.
radioactive materials released by
rupture at 1F2, had been
transported towards north-east by the wind of south-west. Although 131I with a halflife of 8 days was predominant in an early stage, 134Cs (2.06y), 137Cs (30y) and
129mTe (33.6d) are main now. 137Cs with a half-life of 30 years will have been mainly
a target of cleanup in the future.
The radioactive materials absorbed to the particles in the air are detected by the
dust sampling in some points in and out the site. 89Sr (50.5d), 90Sr (29y), 234U and
235U are detected in soil within the range of 20km from the site. The value of these
elements have the range between 1/10 – 1/10,000 of that of Cs. These elements
may be an evidence of the fact that these were released in this accident because the
half-life of 89Sr is as short as 50.5 days. Moreover 140La, 95Nb and 110mAg are detected
slightly in soil towards north-west with a distance of 30km from the site. A small
amount of Pu is detected within the site and it is likely to be released in this
accident. MEXT says that the difference of behavior of these elements might make
the wide range of detected value and more detailed investigation is needed.
The amount of released Sr and Pu is estimated to be extremely smaller than that of
the Chernobyl accident in which contamination by Sr and Pu was severe problem.
Specifically, the highest value of 90Sr/137Cs was 8.2 % at Soma-city but in average, it
is about 0.37 % regardless of the location [16], shown in Fig. 14. Simple scaling of
the radiological equivalence of averaged 90Sr release yields about 0.7 PBq from both
18
analysis in §4.1 and §4.2.2 (=10 PBq x 0.37 % x 20), that is much smaller than that in
Chernobyl accident of 160 PBq (131I-eq.), where 90Sr/137Cs was 1/10.
Monitoring of radioactivity under the sea has been executed. Although
concentration of radioactivity at a sampling point within the harbor of the plant is
high because highly concentrated radioactive water was released from the concrete
near the sluice gate of 1F2 (i.e., 131I 2.8 x 1015 Bq, 134Cs 9.4 x 1014 Bq, 137Cs 9.4 x1014
Bq), that of the outside plant, especially in the area with a distance of more than
30km from the plant, is low[6].
Mechanism of soil contamination by Cs depends on the fraction of it absorbed on the
outer surface of minerals or on the layer structure of clay. While an effective method
for desorption of Cs from clay has not been found yet and it will be expected in the
future, various ways for cleanup of paddy soils should be taken as for the level of
contamination which are stripping surface soil, elimination of clay particles by plow
and vegetation of some plants. Effective decontamination way for soil, which
contains the way for disposal of second-waste, is indispensible for evacuated
residents to return to their home if evacuation zone will be released. For secondary
waste from decontamination, temporary keeping, interim storage and final disposal
are required depending on radiation level. For determining sited for these,
communication with stakeholders, people living there, local government and central
Government, are very important. Academic societies should play important roles by
supplying scientific information on RI behavior and safety evaluation for storage
and disposal.
Summary and Conclusion
We have seen that the release of the radionuclides is subject to the physical and
chemical properties and composition of the fuel core that is highly dependent on its
temperature. By assuming the fraction of the release to the environment, source
term can be evaluated. The integrated source term can also be evaluated
alternatively based on the radiation mon-itor by assuming the fraction of the land
deposition, or by making use of the atmospheric simulation. Although the exact
value of the radioactive release has considerable ambiguity, the amount of the
release is roughly consistent with each other, and is considerably smaller than that
in Chernobyl accident.
Atmospheric diffusion/transport mechanist of each nuclide has not fully understood
yet. However, in the present situa-tion, Cs is the most serious radionuclides to be
considered and the other nuclides may have minor effect on the environ-ment. The
environmental behavior of each species are still needed to be investigated from both
scientific and political points of the view to find a better roadmap of the
decontamination procedures.
19
Fig. 15
Acknowledgement:
The authors wish to acknowledge Dr. Takuji Oda for providing the data based on
the ORIGEN code, and Ms. Ayumi Ito for the support of compiling data.
References:
[1] A.G. Groff, Nuclear Technology, 62 (1983) 335-352.
[2] R.A. Lorenz, M.F. Osborne, A Summary of ORNL Fission Product Release Test
with Recommended Release Rates and Diffusion Coefficients, Oak Ridge National
Laboratory, May 1995 (NUREG/CR-6261).
[3] K. Vierow et al., Nuclear Engineering and Design, 234 (2004) 129-145.
[4] Frank Pasquill, "Atmospheric Diffusion", Ellis Horwood Ltd , 2nd Rev. (1974).
[5] http://monitoring.tokyo-eiken.go.jp/monitoring/f-past_data.html
[6] Nuclear Emergency Response Headquarters Government of Japan, "Report of the
Japanese Government to the IAEA Ministerial Conference on Nuclear Safety - The
Accident at TEPCO’s Fukushima Nuclear Power Stations - " June 2011.
(http://www.kantei.go.jp/foreign/kan/topics/201106/iaea_houkokusho_e.html)
[7] http://www.cpdnp.jp/eng/English/
[8] Japan Chemical Analysis Center, http://www.jcac.or.jp/senryoritu_kekka.html
[in Japanese]
[9] http://radioactivity.mext.go.jp/ja/1100/2011/04/1305284_0421.pdf
[10] http://www.pref.fukushima.jp/j/schoolmonitamatome.pdf
[11] Hideyuki KAWAMURA, Takuya KOBAYASHI, Akiko FURUNO et al., "Preliminary
Numerical Experiments on Oceanic Dispersion of 131I and 137Cs Discharged into
the Ocean because of the Fukushima Daiichi Nuclear Power Plant Disaster", J.
Nuc. Sci and Tech, 48, 1349–1356 (2011).
[12] Yu Morino, Toshimasa Ohara, and Masato Nishizawa, " Atmospheric behavior,
deposition, and budget of radioactive materials from the Fukushima Daiichi
nuclear power plant in March 2011 "GEOPHYSICAL RESEARCH LETTERS, VOL.
38, L00G11 (2011).
[13]
http://www.mext.go.jp/b_menu/shingi/chousa/gijyutu/017/shiryo/__icsFiles/a
fieldfile/2011/09/02/1310688_1.pdf
[14]
http://www.mext.go.jp/b_menu/shingi/chousa/gijyutu/017/shiryo/__icsFiles/a
fieldfile/2011/09/02/1310688_2.pdf
[15] Tokyo Metropolitan Institute of Public Health, http://monitoring.tokyoeiken.go.jp/monitoring/f-past_data.html
[16]
http://www.mext.go.jp/b_menu/shingi/chousa/gijyutu/017/shiryo/1311753.ht
m [in Japanese]
20
Appendix. A Vapor pressure of water
Water tends to condense more at higher pressures. The saturated vapor Psat [atm]
above 1 atm can be empirically approximated as a function of the temperature T
[ºC] by the Antoine equation proposed in 1888,
log Psat =c0 -c1/(c2+T)
(A1)
where {c0, c1, c2} are {8.07131, 1730.63, 233.426} for 1 < T < 100, while {8.14019,
1810.94, 244.485} for 99 < T < 374.
The result of the T as a function of Psat is shown in Fig. 15. For example, sudden
decrease of the pressure, say from 70 to 1 atm, by valving on the SRV, leads to the
sudden drop of the vaporization temperature from 290 to 100 ºC. As a result quite
rapid evaporation of the water in RPV might happen. Therefore, water injection
needs to be started at the same time as, or at least as soon as the SRV opens.
Figure 15 Saturated vapor pressure
21
Appendix B Ground shine
Half-value layer
External exposure from the contaminated ground is called "ground shine". Required
database is compiled in ref. [B1]. Due to the absorption/scattering by the air, the
radiation was attenuated. The characteristic lengths at which the radiation become
half, half-value layer (HVL), of lead, iron, aluminum, water, air and concrete are
compiled in Table E2 [B1].
Roughly speaking, the dose rate by the ground shine reflects the area inside the
circle of several times the air HVL in radius. Each point of Figs. 10 and 14 represents
at least 4.57 and 4 km2, respectively. Therefore, the influence of the area for the
adjacent points in the MEXT monitoring that could cause a double-counting of the
dose rate are eliminated.
Dose factor
Conversion factors from [Bq/m2] to [Sv/h] listed in Table E3 [B1] include effective
dose rate for external dose and committed inhalation due to resuspension resulting
from remaining on contaminated ground. However, since the inhalation dose for the
present situation is considerably small, this database can also be used for the pure
external dose.
The shielding factor (SF) needs to be considered, since the evaluation above is the
ideal case where the ground is smooth spread over the infinite disk. In the ordinary
ground ref[B1] proposed to use SF of 0.47-0.85(representative of 0.7).
In addition, the dose factors for the plume submersion, that is crucial in the early
stage of accidents, is calculated in the Table III.1 of ref.[B2].
Radiological equivalence
Radiological equivalence is the ratio of the activity released of a specified
radionuclide to the case for 131I. This value is used to classify the scale of the
accident as describe in ref [B3]. It consider the above mentioned ground
contamination and the plume submersion. The database for the total effect on the
public are given in Table 15 in Appendix I of ref.[B3].
These are listed in Table B1 for species of interest. Although 134Cs is the present
leading radiator, 137Cs is the most crucial nucleus in the evaluation of the rating of
the accident. On the other hand, 90Sr is less effective due to both the low
concentration (approximately below 1 % of 137Cs) and the low radiological
equivalence (half of 137Cs).
22
Experimental determination of the Shielding Factor
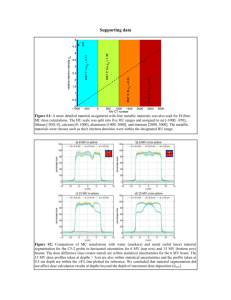
By comparing the ground
contamination with the dose
rate for each point in the
radiation MAP, shielding
factor SF can also be
evaluated, as shown in Fig.
16.
In the fitting procedure, we
fixed the baseline
corresponding to the
background natural
radiation dose.
One can see that the SF=0.7
is a plausible value in the
Figure 16 Measured dose rates vs. those evaluated from the
ground shine conversion with the ideal case, SF =1. Background
general discussion, but the
dose rate of 0.06 µSv/h was assumed.
scattering of the data is
considerably large.
Therefore, it may mislead if one calculate the external exposure from the local
ground contamination. On the other hand, dose rate was the averaged, namely
effective, value around the measurement point.
[B1] IAEA, "Generic procedures for assessment and response during a radiological
emergency" [IAEA-TECDOC-1162] (2000). http://wwwpub.iaea.org/mtcd/publications/pdf/te_1162_prn.pdf
[B2] Keith F. Eckerman and Jeffrey C. Ryman "External exposure to radionuclides in air,
water, and soil" FEDERAL GUIDANCE REPORT NO. 12, EPA-402-R-93-081, OAK
RIDGE NATIONAL LABORATORY (1993).
http://nnsa.energy.gov/sites/default/files/seis/fgr12.pdf
[B3] INES, The International Nuclear and Radiological Event Scale "the international
nuclear and radiological event scale user’s manual 2008 edition" (international atomic
energy agency vienna, 2009)" http://www-pub.iaea.org/MTCD/publications/PDF/INES2009_web.pdf
Air
[Sv/h]/[Bq/m2]
I-131 eq.
HVL[m]
I-131
1.30E-12
1
8.0d
55.9
Cs-134 2.06y 71.9
5.40E-12
2.8
Cs-137 50y
69.2
2.10E-12
40
Sr-89
50.5d 80.5
8.0E-15
0.5
Sr-90
29.1y 1.0E-15
20
Table 5 Dataset to access radiological equivalence to I-131
nucleus
τ
23