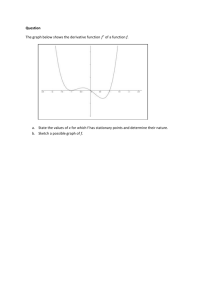
Mehta P et al Biochem transactions 2014 - Spiral
advertisement

1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Biochemical Society Transactions Is the cellular and molecular machinery docile in the stationary phase of Escherichia coli? Parul Mehtaa,1, Goran Jovanovica, Liming Yingb, Martin Bucka,1. a Department of Life Sciences, Imperial College London, London SW7 2AZ, United Kingdom; National Heart and Lung Institute, Imperial College London, London SW7 2AZ, United Kingdom b 1 Corresponding authors, Martin Buck, Department of Life Sciences, Faculty of Natural Sciences, Imperial College Road, Imperial College, London SW7 2AZ, UK. Tel: +44 (0)207594-5442; E-mail: m.buck@imperial.ac.uk; Parul Mehta, Division of Cell and Molecular Biology, Department of Life Sciences, Imperial College London, London SW7 2AZ, United Kingdom Tel: +44 (0)207-594-5366; E-mail: p.mehta10@imperial.ac.uk. Keywords: stationary phase, Phage shock protein response (Psp), membrane stress, cytoplasmic crowding, bacterial enhancer binding protein Abbreviations: Phage shock protein response (Psp), logarithmic phase (log), innermembrane (IM), Escherichia coli (E. coli), bacterial enhancer binding protein (bEBP) Abstract The bacterial cell envelope retains a highly dense cytoplasm. The properties of the cytoplasm change with the metabolic state of the cell, the logarithmic phase being highly active and the stationary phase metabolically much slower. Under the differing growth phases many different types of stress mechanisms are activated in order to maintain cellular integrity. One such response in enterobacteria is the Phage shock protein (Psp) response that enables adaptation to inner membrane (IM) stress. The Psp system consists of a transcriptional activator PspF, negative regulator PspA, signal sensors PspBC and PspA and PspG acting as effectors. The single molecule imaging of the PspF showed the existence of dynamic communication between the nucleoid-bound states of PspF and membrane via negative regulator PspA and PspBC sensors. The movement of proteins in the cytoplasm of bacterial cells is often by passive diffusion. It is plausible that the dynamics of the biomolecules differs with the state of the cytoplasm depending on the growth phase. Therefore, the Psp response proteins might encounter the densely packed glass-like properties of the cytoplasm in the stationary phase, which can influence their cellular dynamics and function. By comparing the properties of the log and stationary phases, we find that the dynamics of PspF are influenced by the growth phase and may be controlled by the changes in the cytoplasmic fluidity. Bacterial cells, their different growth phases and the subsequent cellular changes Bacterial growth involves four different growth phases lag, logarithmic (log), stationary, and death phase [1]. The bacterial growth dynamics can be followed by plotting cell growth (absorbance at optical density of 600) versus incubation time and is exponential (log phase) after leaving lag phase in balanced growth [1, 2]. Each of the bacterial growth phases has its own characteristic feature, for example the log phase is highly productive phase with active metabolism, rapid cell division and displays cellular homogeneity [1, 3]. On the other hand the bacterial cell undergoes a number of structural as well as physiological changes when in stationary phase, including a decrease in cell volume, cell shape changes, constriction of nucleoid, alterations in cell wall composition and accumulation of storage materials [3]. The extracellular environment especially the growth medium of bacterium also changes under stationary phase, it becomes more basic because of the switch in bacterial lifestyle from 1 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 carbohydrate metabolism to consuming more amino acids [1]. Recently detailed studies on the properties of the cytoplasm revealed that the bacterial cytoplasm has glass-like properties, which are specifically perceived by molecular complexes [4]. Such glass-like properties are dependent on the metabolic states of the cell, and with the late-stationary phase glass-like properties of the cytoplasm are exaggerated (see figure 1) [4]. Phage Shock response in Escherichia coli Many enterobacteria reside in harsh environmental conditions such as the human gut and challenging rhizospheres where they come across different stresses [5, 6]. In order to combat wide variety of stresses bacteria like E. coli have evolved different types of responses. One highly specific response to inner membrane (IM) stress is Psp response; Psp system mounts an adaptation to IM stress, by repairing the IM damage and consequently conserving the proton motive force and energy production [5]. Induction of Psp response is unique in the sense that it is dependent on the alternative sigma factor σ54, and requires a specific activator, bacterial enhancer binding protein (bEBP) [5]. The bacterial 54 systems are of general importance because the transcription mechanism displays resemblance to eukaryotic transcription using RNA polymerase II and many of the σ54 promoters drive expression of genes involved in biological stress responses such as pathogenicity, persistence and even biogeochemical cycles [5]. The Psp response is induced by wide array of external stimuli such as mislocalisation of outer-membrane secretin such as pIV, iononphores, fatty acids, ethanol, and impaired protein secretion pathways [5, 6]. In E. coli PspF as an ATPase competent hexamer activates the transcription of physically distant pspA-E operon and pspG gene under stress inducting conditions (see Figure 2). The low-order oligomers of PspA bind and negatively regulate PspF activity under non-stress conditions while upon IM stress functions as a higher-order oligomer to recover IM functionality [7, 8]. The cellular landscape of the Psp response in E. coli A detailed knowledge of PspF and PspA localisations and their self-associations is a key to establishing how the Psp response is controlled and functions. With the help of millisecondresolution single molecule fluorescence microscopy in live E. coli cells we observed the fluorescently tagged (with Venus fluorescent protein) PspF and PspA proteins under nonstress and IM stress conditions [7,8]. With the imaging studies we could elucidate the mechanisms of control and activation of the Psp response in intact native cell environment [7]. The spatial localisations, 2D dynamics and functional stoichiometry of the diffraction limited, localised spots of fluorescent fusion proteins of PspF and PspA were determined. The repressive PspF-PspA complex bound to psp promoter(s) on the DNA dynamically and transiently communicates with the IM under non-stress conditions. Under IM stress conditions PspA dissociates from the PspF-PspA inhibitory complex and binds the stressed membrane as a higher order oligomer [7, 8, 9]. Under all experimental conditions the PspF assembled and localised on the DNA as a single hexameric species, binding and activating a single psp promoter (pspA or pspG) at a time [7]. The two different functional states of PspF were distinguished by cellular 2D diffusion coefficients. The PspF actively engaged in transcriptional activation under membrane stress conditions displayed (apparent diffusion coefficient = 0.018 µm2/s) seven times slower compared to the PspF within PspF-PspA inhibitory complexes under non-stress conditions (0.134 µm2/s) [7]. The variations in the dynamic behaviour of PspF were attributed to its different functional states and interactions with other participating protein complexes, whereby, PspA (via the PspBC signal sensors) contributes to faster dynamics of PspF such as movement to the membrane. Clearly, the DNA associated diffusion of PspF changes with the imposition of IM stress, and other factors influencing the observed cellular dynamics of PspF such as interactions with other protein complexes, nucleoid organisation and even cellular crowding needs further exploring [10]. 2 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 PspF changes its cellular mobility in stationary phase The cellular dynamics and localisation of PspF and PspA reported so far have only been studied in log phase E. coli cells. Here we set out to assess how the cellular landscape of Psp response changes in early stationary phase under non-stress and stress conditions. The survival of cells in the stationary phase irrespective of accumulating toxins, energy efficient lifestyles and elevated pH levels of the extracellular environments requires life-style adaptions. The cytoplasm crowding is probably exaggerated in the stationary phase with the reduction in relative cell size and volume. Since the glass-like properties of the cytoplasm are also pronounced with stationary phase, there is a possibility that the cellular mobility of the repressive PspF-PspA complex under non-stress conditions and active PspF under IM stress are reduced. Our direct measurements of apparent 2D diffusion coefficients in early stationary phase showed that the PspF self assemblies were almost immobile bound to the DNA (apparent diffusion coefficient = 0.009 µm2/s), there was reduction in the apparent diffusion coefficient values from the PspF assemblies in the log phase under non-stress conditions (unpublished work P.Mehta, G.Jovanovic, L.Ying and M.Buck). In addition to the reduction in PspF mobility, the number of DNA-localised diffraction limited foci increased from single foci to two foci per chromosome, the cell length was reduced and the relative protein density increased by 33% in stationary phase as compared to log phase (unpublished work unpublished work P.Mehta, G.Jovanovic, L.Ying and M.Buck). The explanation for slower cellular dynamics and more PspF foci/cell include the possibilities that the binding of PspF to its specific pspA and pspG promoters is more stable and prolonged in the stationary phase than in log phase. These changes in dynamics and number of foci could be linked to changes in DNA supercoiling impacting upon transcription factor binding, including that of PspF, and its target closed promoter complex. As in stationary phase the nucleoid DNA or plasmid DNA exists in a more relaxed state, which might affect the DNA binding ability of the proteins [12]. Concluding remarks Single molecule fluorescence microscopy of chromosomally encoded fluorescently tagged PspF and PspA fusion proteins offers new mechanistic insights into the control of the Psp response at the cellular level. The mobility and localisation of the PspF activator and the PspA negative regulator/effector proteins and their complexes in log phase live E. coli cells revealed the surveillance mode of the PspF-PspA complex for IM damage [7]. In the stationary phase, the localisation and dynamics of PspF changes suggesting some longer lived DNA interactions. These have the potential to impact upon the kinetics of stress response induction, and the degree of cell to cell variability expected from inherently stochastic events which occur during transcription such as bursts in gene expression and phenotypic variation between genetically identical cells. Each of these aspects of gene expression and phenotype can now be explored. The application of advanced data analyses and mathematical modelling has enabled to characterise complex phenomenon (such as redefining of cytoplasmic properties) [4, 11]. Being able to analyse changes in protein behaviour in the different growth phases of bacteria at the single molecule level will not only expand our understanding of bacterial cell biology. It will also enable to understand the survival mechanisms developed by bacteria in order to flourish in harsh growth-limiting conditions [13]. Acknowledgements This work was supported by project funding from the Leverhulme Trust. References 1) Farrell, M.J. & Finkel, S.E. (2003) The growth advantage in stationary phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment. J. Bacteriol. 185, 7044-7052. 3 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 2) Whitman, W.B. (2009) The Modern Concept of the prokaryote. Journal of Bacteriology. 191, 2000-2005. 3) Makinoshima, H., Aizawa, S., Hayashi, H., Takeyoshi., M. et al. (2003) Growth phasecoupled alterations in cell structure and function of Escherichia coli. Journal of Bacteriology. 185, 1338-1345. 4) Parry, B.R., Surovtsev, I.V., Cabeen, M.T., O’Hern, C.S., et al. (2014) The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell. 156, 183194. 5) Joly, N., Engl, C., Jovanovic, G., Huvet, M., et al. (2010) Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiology Reviews. 34, 797-827. 6) Darwin, A.J. (2005) The phage-shock-protein response. Molecular Microbiology. 57, 621628. 7) Mehta, P., Jovanovic, G., Lenn, T., Bruckbauer, A., et al. (2013) Dynamics and stoichiometry of a regulated enhancer-binding protein in live Escherichia coli cells. Nature Communications. 4:1997, 1-9. 8) Jovanovic, G., Mehta, P., McDonald, C et al. (2014) The N-terminal amphipathic helices determine regulatory and effector functions of phage shock protein A (PspA) in Escherichia Coli. J. Mol. Biol. 426, 1498-511. 9) Jovanovic, G., Mehta, P., Ying, L., Buck, M (2014). Anionic lipids and the cytoskeletal proteins MreB and RodZ define the spatio-temporal distribution and function of membrane stress controller PspA in Escherichia coli. Microbiology. In press 10) Mika, J.T. & Poolman, B. (2011) Macromolecule diffusion and confinement in prokaryotic cells. Current Opinion in Biotechnology. 22, 117-126. 11) Golding, I. & Cox, E. (2006) Physical Nature of Bacterial Cytoplasm. Physical Review Letters. 96, 098102 1-4. 12) Reyes-Dominguez, Y., Contreras-Ferrat, G et al. (2003). Plasmid DNA supercoiling and Gyrase activity in Escherichia coli wild-type and rpoS stationary-phase cells. J. of Bacteriol. 185, 1097-1100. 13) Finkel, S.E. (2006). Long-term survival during stationary phase: evolution and the GASP phenotype. Nature Rev. Microbiol. 4, 113-120. Figure legends 4 195 196 197 198 199 200 201 Figure 1 Schematic of dense bacterial cytoplasm: The figure illustrates the possible constraints in the cellular mobility of the phage shock protein F (PspF) assembly when communicating with other proteins like phage shock protein A (PspA), RNApolymerase holoenzyme with σ54, enhancer DNA sites or with membrane in the latestationary phase with denser cytoplasm and more stored materials. The main mode of movement in the cell for protein complexes is by passive diffusion [4, 10]. 5 202 203 204 205 206 207 Figure 2 Schematic of Phage Shock Protein (Psp) Response The schematic represents the possible cellular landscape of the Psp response showing the different spatial localisation and respective function of Psp proteins. PspF functions as a bacterial enhancer binding protein activating σ54 dependent transcription from either the pspA promoter or pspG promoter. PspA is a dual function protein, acting as a repressor of PspF during non-stress conditions and as an effector to help maintain membrane integrity under stress conditions [7]. 6