Blue Light Cystoscopy with Cysview Fact Sheet
advertisement

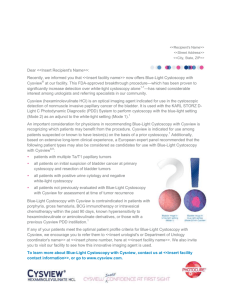
Blue Light Cystoscopy with Cysview®(hexaminolevulinate HCI) for Bladder Cancer About Bladder Cancer Detection Bladder cancer is the sixth most commonly diagnosed cancer in the United States and has the highest recurrence rate of any form of cancer.1 Doctors who suspect patients may have bladder cancer can use a procedure called cystoscopy to obtain images of tumors in the urinary bladder.2 Traditional cystoscopy involves a cystoscope with a lens and a white light for visualizing suspicious lesions. Once lesions are identified, doctors can surgically remove cancerous tissues through a procedure called a transurethral resection of the bladder tumor (TURBT).2 However, an estimated 10 to 20 percent of bladder tumors are overlooked or not visible using this traditional form of white light cystoscopy, which may mean doctors aren’t removing all of the cancer during a TURBT.3 About Cysview® In patients that are suspected or known to have lesions based on prior cystoscopy, Blue Light Cystoscopy with Cysview is approved by the FDA for the detection of non-muscle invasive papillary cancer of the bladder. Cysview is delivered into the bladder about an hour prior to the cystoscopy and is absorbed by cancerous tissue, exploiting the fluorescent properties of naturally occurring molecules in malignant tissues.4 Using Cysview with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD)™ device, doctors are able to view comparative white and blue images that may make hard-to-see tumors more visible (see below), and thereby easier for the doctor to identify and remove.5,6 WHITE LIGHT CYSTOSCOPY BLUE LIGHT CYSTOSCOPY AS AN ADJUNCT TO WHITE LIGHT Clinical Data of Cysview In a clinical study of 814 patients, one or more additional Ta or T1 bladder cancer lesions were detected by Cysview in 16.4% of the patients compared to white light alone.5 Blue light cystoscopy supports a more complete resection and has been reported as improving oncological patient outcomes, reducing recurrence rates by approximately 20 percent, and prolonging recurrence-free survival.7 Based on a combination of clinical data and economic analysis, a study showed that the five years costs for patients who initially receive blue light cystoscopy as part of their TURBT were lower than those patients who initially receive white light TURBT.7 Blue light Cystoscopy with Cysview can easily be incorporated into clinical practice. To date more than 200,000 patients has received this innovation world wide Important Safety Information Cysview is not a replacement for random bladder biopsies or other procedures used in the detection of bladder cancer and is not for repetitive use. Anaphylaxis reactions including anaphylactoid shock, hypersensitivity reactions, bladder pain, cystitis, and abnormal urinalysis have been reported after administration of Cysview. The most common adverse reactions seen in clinical trials were bladder spasm, dysuria, hematuria, and bladder pain. Cysview should not be used in patients with porphyria, gross hematuria, or with known hypersensitivity to hexaminolevulinate, or in patients receiving intravesical chemotherapy or BCG treatment within 3 months of Cysview photodynamic blue-light cystoscopy. There are no known drug interactions with hexaminolevulinate; however, no specific drug interaction studies have been performed. Using Cysview, fluorescence of non-malignant areas may occur, and Cysview may fail to detect some malignant lesions. Safety and effectiveness have not been established in pediatric patients. Cysview should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus. It is not known whether hexaminolevulinate is excreted in human milk. Because many drugs are excreted in human milk, exercise caution when Cysview is administered to nursing mothers. No clinically important differences in safety or efficacy have been observed between older and younger patients. Cysview is approved for use with the Karl Storz D-Light C Photodynamic Diagnostic (PDD) system. For system set up and general information for the safe use of the PDD system, please refer to the Karl Storz instruction manuals for each of the components. Prior to Cysview administration, read the Full Prescribing Information and follow the preparation and reconstitution instructions. Full Prescribing Information is available here. (2013) General Information. Bcan.org Retrieved July 8, 2013 from http://www.bcan.org/facing-bladder-cancer/frequently-askedquestions/general-information Witjes, Alfred & van der Heijden, Antoine. (2009) 556–562. Recurrence, Progression, and Follow-Up in Non–Muscle-Invasive Bladder Cancer. European Association of Urology. Retrieved July 9, 2013 from http://euacme.org/europeanurology/upload_articles/van%20der%20Heijden%2 0Sept%20Supp.pdf. Kausch I, Sommerauer M, Montorsi F, et al. Photodynamic diagnosis in non–muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol. 2010;57(4):595606. Cysview [prescribing information]. Princeton, NJ: Photocure ASA; 2011. Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010;184(5):1907-1913. Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol. 2007;178(1):68-73. Sievert, K.D et al. (6/27/2009). Economic aspects of bladder cancer: what are the benefits and costs? World Journal of Urology. Retrieved on July 9, 2013 from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2694315/