STAT 115: SECTION 11
Research Example
Evaluation of a urine test for detection of bladder
cancer
Urine Test for Bladder Cancer
THE JOURNAL OF UROLOGY® Vol. 188, 741-747, September 2012
Bladder Cancer
http://www.urologyhealth.org/urology/articles/images/anatomy_bladdercancer.jpg
Bladder Cancer
www.cancer.gov/cancertopics/types/bladder
Detection of bladder cancer
Standard practice for detection of bladder cancer includes:
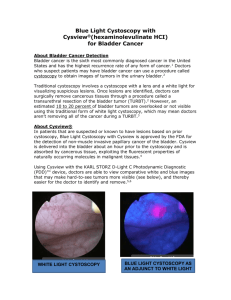
Cystoscopy
• a cystoscope hollow tube with a lens at the end is passed through the
ureter and into the bladder
• Can be flexible or rigid
• Used to visualise the inside the bladder to see if there are any abnormal
areas
• Abnormal areas are biopsied – a small piece of tissue is removed and sent
to the pathologist to determine if there is cancer
Cytology:
• Cells in a urine sample are looked at to see if there are any abnormal cells
that might have been washed off the bladder wall.
Other urine tests:
eg Nuclear Matrix Protein 22 (NMP22™)
Performance of a diagnostic test
Sensitivity measures how good the test is as identifying people with disease
measured on a group of people with the disease
Specificity measures how good the test is in ruling out disease in people who
don’t have disease
measured on a group of people without the disease
Continuous diagnostic tests
Test which gives a continuous measure
Disease
Disease free
Increased
specificity
Increased sensitivity
Test negative
Cut-off
Test positive
Detection of bladder cancer
Cystoscopy in combination with biopsy and histopathological diagnosis:
Sensitivity of over 90%
Specificity of 100%
Although examination with flexible instruments is considered to be a
rapid routine procedure, cystoscopy is invasive and associated with a cost.
Urinary cytology:
Sensitivity 34%
Specifcity 99%
Urine cytology is also relatively expensive and requires an experienced
pathologist and meticulous sample collection, storage and preparation.
Regardless, it is still in routine use as an adjunct to cystoscopy.
Development of urine test: Phase I
Clin Cancer Res 2008;14(3)
Original development of the test
• identified 4 RNA markers CDC2, MDK, IGFBP5, and HOXA13, which
distinguished TCC and non-cancer tissue
• used statistical methods to combine these continuous marker
variables into a single score (uRNA-D®) which discriminated
between bladder cancer and non-bladder cancer samples
Continuous diagnostic tests
Test which gives a continuous measure
Disease
Disease free
Increased
specificity
Increased sensitivity
Test negative
Cut-off
Test positive
Development of urine test: Phase I
Urine samples were obtained from
• 75 bladder cancer patients (with Transitional Cell Carcinoma)
• 77 patients with nonmalignant diseases or other cancers, in whom
bladder tumors were excluded by flexible cystoscopy.
• The uRNA-D® test consists of qRT-PCR reactions for the four RNA markers
MDK, CDC2, HOXA13 and IGFBP5. Each of these markers is up-regulated
in transitional cell carcinoma (TCC).
• These early studies found for cut off fixed at a specificity of 85%
• Sensitivity = 48/75 = 60%
• Stage Ta; sensitivity = 48%,
• Stage T1; sensitivity = 90%,
• Stage >T1: Sensitivity= 100%
Development of diagnostic test: Phase II
THE JOURNAL OF UROLOGY® Vol. 188, 741-747, September 2012
Development of diagnostic test: Phase II
Primary Objective:
To determine the characteristics (sensitivity, specificity, area under the ROC
curve, positive and negative predictive values) of the uRNA-D® test for the
detection of TCC in patients with a recent history of gross haematuria.
Design:
• Cohort study
• Participants: patients with a recent history of gross haematuria, who are
undergoing investigation of haematuria (by cystoscopy) for possible
urological cancer.
• Eligible consenting patients provided a freshly voided mid-stream urine
sample prior to cystoscopy, for uRNA®, the NMP22™ tests and urine
cytology analysis.
The presence of urinary tract TCC was determined by biopsy and
histopathological examination within a 3 months
Results of Phase II
A number of the 594 patients registered excluded from analysis
• inadequate determination of TCC status (n=18):
• lack of availability of urine test (n=13):
Figure 5. Test results for uRNA-D® by bladder cancer status and by
area
Figure 6. Test results for NMP22 ELISA by bladder cancer status
and area.
Results of Phase II
Using data from New Zealand and Australia only
Sensitivity (at specificity of 85%):
Number with bladder cancer = 66
Number with a positive uRNA-D test = 41
Sensitivity = 41/66= 0.621 or 62.1%
Figure 7. Comparison of ROC curves for uRNA-D® and NMP22
ELISA for New Zealand and Australia
.
Figure 16. ROC curve for Cxbladder® (a refinement of the
uRNA-D test based on the cohort study data)
.
Confidence interval calculated using an exact method, so is slightly wider
than the method taught in this class, which was