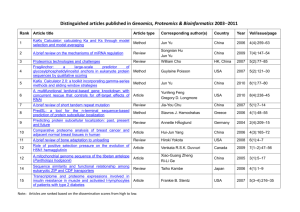
Proposta di ricerca: 1. State of the art Anthracyclines were the first
advertisement

Proposta di ricerca: 1. State of the art Anthracyclines were the first extremely powerful and highly versatile anticancer agents discovered by Farmitalia Research Laboratories in the 1950’s. These “old” molecules today are still the most effective wide spectrum antineoplastic antibiotics employed in chemotherapy. They also represent the most prominent example when Italian pharmaceutical research has made a significant improvement in worldwide therapeutic practice. Although active against a wide variety of solid tumours and haematological malignancies, clinical use of anthracyclines is hindered by tumour resistance and toxicity (e.g. cardiotoxicity) to healthy tissue. Recent progress in the conjugation of anthracyclines with different types of carriers holds the promise to decrease the side-effects and to improve selectivity.(1) Success of the design of next generation anticancer drug however highly depends on our current knowledge of the mechanisms responsible for the cellular uptake and the cytotoxic and apoptotic effects. A recent high-throughput proteomic work analyzed the changes occurs in the entire proteome level of HepG2 upon doxorubicin treatment.(2) The action mechanism proposed to be targeted to the nucleus, mitochondria and cell membrane reorganization. This is in agreement with our preliminary quantitative proteomic data obtained on the HL-60 model system by iTRAQ assay and MuDPIT analysis.(3) The expression level of 21 proteins was shown to change significantly upon daunomycin treatment at IC25 value. In particular, nucleolin and nucleophosmin abundant p53 binding proteins - known to be over-expressed in malignant tumors and relapsed/refractory acute leukemia - were found to be significantly down-regulated. We found that protein DJ-1, a multifunctional protein that has been implicated in tumor pathogenesis and up-regulated in acute leukemia was also down-regulated. While the analyses of the entire cell proteome give very valuable elements for the better understanding of drug mechanism of action in general, it gives only limited information on the mechanisms of drug-conjugate delivery. Therefore, here we put forward a proteomic study which specifically targets three compartments: the cell-surface, extracellular and nuclear sub-proteomes. This shall provide us a higher resolution picture on the changes in the expression levels of those proteins which are directly involved in the drug-uptake and apoptotic action with the final aim to aid the design of new efficient drug carrier bioconjugates. 2. Motivation for co-operation, expertise and resource The two research groups, the Mass Spectrometry and Proteomics Laboratory at the Institute of Protein Biochemistry (IBP) of CNR in Naples (Italy) and the Research Group of Peptide Chemistry, Hungarian Academy of Sciences (HAS) at Eötvös Loránd University (RGPC) in Budapest (Hungary) are working in complementary research fields and have a long standing collaboration. Both groups interested in i) protein/peptide structural analysis, ii) quantitative analysis of protein expression level, iii) characterization of changes in protein posttranslational modifications and iv.) rational design of polypeptide/protein carriers. We have participated with success in the “Programma triennale 2010/2012” financed by CNR/MTA agreement with joint project entitled “Quantitative proteomics for the identification of differentially expressed proteins in cells treated with daunomycin bioconjugates”. This gave us the opportunity to exchange researchers and to train Ph.D. students through active participation in the project. In addition, this project led to interesting scientific results in the field of proposed research (see final report). The researchers at IBP-CNR involved in the proposal have a strong background in the application of mass spectrometry in proteomics and biochemistry and has a well-equipped laboratory for nano-scale proteomic studies. The group recently published studies on the characterization of transport mechanisms through proteomic analysis of cell surface and extracellular proteins.(4-5) In addition, recently a patent application has been made for the isolation of extracellular vesicles and their use in clinical biomarker discovery.(6-7) The group has also experience in the characterization of phosphorylation status of proteins. Recently they developed a selective phosphopeptide enrichment method streamline applicable to shotgun proteomics.(8) The Research Group of Peptide Chemistry, HAS at Budapest, on the other hand, has an outstanding international reputation in polypeptide synthesis, in the preparation and characterization of peptide bioconjugates (9-11) and in peptide and protein chemistry (12) in general. The RGPC has been updated by cell culture and flow cytometry laboratories mandatory for the present proposal. In addition, the RGPC has been collaborating with Dr. Pocsfalvi since a long time. This collaborative research has resulted in 11 joint publications and 16 communications at international conferences. In particular Dr. Schlosser visited several times the mass spectrometry laboratory at ISA-CNR in Italy and her research activity was financed by different agencies. During her visits in Italy Dr. Schlosser had the opportunity to learn and practice principal and sophisticated proteomic methods and to apply it to a number of collaborative scientific projects. Available resources: Proteomics related scientific instruments including a two dimensional nano HPLC system (LCPackings) and a triple quadrupole time of flight mass spectrometer equipped with a nano-ESI source (QStar-Elite), a surface enhanced laser desorption (SELDI)-TOFMS, HPLC systems and robotics are available at IBP-CNR. All the necessary equipment for cellular biology including flow cytometry as well as laboratories and equipment for synthetic chemistry are available at ELTE-MTA. Human resources: In this proposal we put together two research teams including researchers and students at different levels of their carriers with high level of interdisciplinarity and complementary research interests. Grants of Ph.D. students have been obtained separately but their activity will be complementary and compatible with their Ph.D. works. 3. Workplan During the three year period, research activities will be organized in the following stages: 4. 1st YEAR: Systematic characterization of the changes in the cell NUCLEAR PROTEOME upon treatment with different daunomycin (Dau)-peptide conjugates. 2nd YEAR: Changes in the cellular SECRETOME to target cellular trafficking of Daupeptide conjugate treated HL-60 cells. 3rd YEAR: Study the influence of different CARRIER MOLECULES on the nuclear proteome, cellular trafficking, and apoptosis in HL-60 cells treated by Daunomycin conjugated to different polypeptide/protein carriers. Chemical, biomolecular and analytical methodologies: First, the drug-peptide bioconjugates will be prepared. HL-60 human leukemia cell line will be used as a model system because daunomycin is widely used in the treatment of leukemia. Time- and concentration-dependency of cellular uptake will be determined by flow cytometry to assign the appropriate treatment time and concentration, which can evoke changes in protein expression profile. This part of the work will be performed at RGPC in Budapest. Cellular sub-proteome fractions (membrane surface, nuclear and extracellular) will be prepared and analyzed by proteomics using two control samples (untreated and Dau-treated cells). Protein expression profiles of control samples will be compared with that of Dau-peptide conjugates treated cells. In-solution proteomics will be applied using MudPIT (multidimensional protein identification technology) with iTRAQ (isobaric tag for relative and absolute quantitation) labeling. In this workflow, protein samples will be first enzymatically digested and then labeled. After iTRAQ labeling samples are pooled and fractioned by two-dimensional nano HPLC using SCX separation in the first and reverse phase C18 chromatography in the second dimension. Separated peptides then will be on-line analyzed by nano-ESI tandem mass spectrometry (MS/MS) which facilitate multiplex quantitative analysis of the four samples. Low molecular mass reporter ions are used to relatively quantify the peptides and the proteins from which they originate. This part of the work will be performed at IBP-CNR (Naples). 5. Expected results and benefits The main expected scientific outcome will be the lists of differentially expressed proteins and phosphoproteins upon treatment using the different drug-bioconjugates as new vehicles to anticancer drug targeting. The rationalization of nuclear, membrane and secreted protein expression profiles will help to better understand apoptotic action, cellular uptake and transport mechanisms. This information is mandatory to improve rational design of drug delivery system which is the ultimate goal of this project. The project by collaborating with other national and international research groups, clinics and SMEs will also serves to put together new ideas and to find new ways to continue our collaboration in the field. We also expect to develop new methods using nanomaterials for phosphoprotein characterization. The results will be published in international journals and newly developed methods (if any) will be patented and applied to solve biochemical and medical problems. The main expected benefit is that both research groups can use the different instrumentations and to learn different approaches from each other’s experience. Both groups can profit from joint results especially in the fields of solid phase affinity chemistry, nano-separation, proteomics and drug carrier bioconjugate design and synthesis. The Hungarian researchers could use HT proteomic instrumentation not yet available in Hungary. Young Hungarian Ph.D. students will be trained for the use of modern mass spectrometric techniques and proteomics not yet available for them. The Italian researchers can learn peptide and bioconjugation chemistry, cell culture, sample preparation methods and techniques to study the surface chemistry process developed in Budapest. They also can use the advanced online library search facility during their visits at the RGPC. The scientific results will be published in international journals and newly developed methods will be applied to solve biochemical and medical problems. Additionally, we aim to organize a consortium to promote EU-FP7 project proposal including the participants of the present application. References: 1. Miklán, Zs. et la. (2009) Cells, Pep. Sci. 92, 489-501.; 2. Hammer, E. et al. (2010) Proteomics, 10, 990-114; 3. Schlosser, G. et a. (2012) (in preparation); 4. Palmieri, G. (2009) Mol Cell Proteomics, 8, 2570-2581; 5. Pocsfalvi, G. (2011) et al. Journal of Proteome Research 10, 5326-5337; 6. Pocsfalvi, G., et al., (2011) Ufficio Italiano Brevetti e Marchi, UIBM, Italy; 7. Raj, D.A.A., et al (2012) 81, 1263–1272; 8. Pocsfalvi, G. (2009 )In Methods in Enzymology, 457, 8196; 9. Szabó, I. et al (2009) Bioconjug. Chem. 20, 656-65; 10. Bánóczi, Z., et al. (2010) Bioconjug. Chem. 2, 1948-1955; 11. Miklán, Zs. et al. J. (2011) Peptide Science, 17, 805-811; 12. TQke, O., et al. (2011) Eur. Biophys. J., 40, 447-462. Obiettivi: The principal aim during the three-year collaborative research is to characterize the changes in specific cellular sub-proteomes induced by treatment with three different daunomycin-containing bioconjugates using a quantitative proteomic approach. The existing long-standing collaboration between the two groups makes feasible to achieve this ambitious goal. The peptide bioconjugates will be prepared and characterized by the RGPC group in Budapest because they have a strong expertise in this field. Considering their different uptake properties three candidate carrier peptides have been chosen for this work: 1. oligoarginine which enters the cell mainly by macropinocytosis, 2. IELLQAR peptide ligand which binds to E-selectin, and 3. gonadotropin-releasing hormone (GnRH), which binds to GnRH receptor. Cellular phenotypes, uptake, cytostatic and apoptotic effect of these conjugates will be monitored in preliminary experiments. Quantitative proteomics will be performed at the Mass Spectrometry and Proteomics Laboratory at IBP-CNR using peptide tagging to measure the differences in protein expressions of Dau-conjugates treated vs. Dau-treated and untreated cells. Based on previous work on the action mechanisms of anthracyclines we propose to perform a targeted study which will enable us to define the changes in sub-proteomes with a higher resolution and a deeper extent than previous global analysis could do. In the first stage we aim to apply quantitative nuclear proteomics which gives the opportunity to monitor protein effectors that contribute to cellular phenotype. In a second step membrane surface and extracellular fractions will be analyzed in order to gain knowledge on cellular trafficking. The later could be particularly of value for the rational design of new carrier molecules. In addition, we also aim to monitor protein phosphorylation status in the nucleus upon bioconjugate-drug treatment using a phosphoproteomic approach recently developed by the group at IBP-CNR.(8) In this context we aim to further develop our method by applying nano-sized ZrO2 particles for the batch-wise enrichment of phosphopeptides. Besides the scientific objectives this project aims to provide high quality training for young Ph.D. students and researchers mainly in the fields of the synthesis and characterization of bioconjugates, cellular biology and proteomics. In particular, the Hungarian researchers could use the state-of-the art instrumentation of IBP-CNR not yet available in Hungary. The Ph.D. students will be trained for the use of nano-scale two-dimensional HPLC and mass spectrometric techniques and their application in quantitative proteomics. The Italian researchers can learn synthetic peptide chemistry, methodologies in cellular biology, bioconjugates synthesis, and techniques to study the surface chemistry process developed in Budapest.