Mole Calculations Review Sheet
advertisement
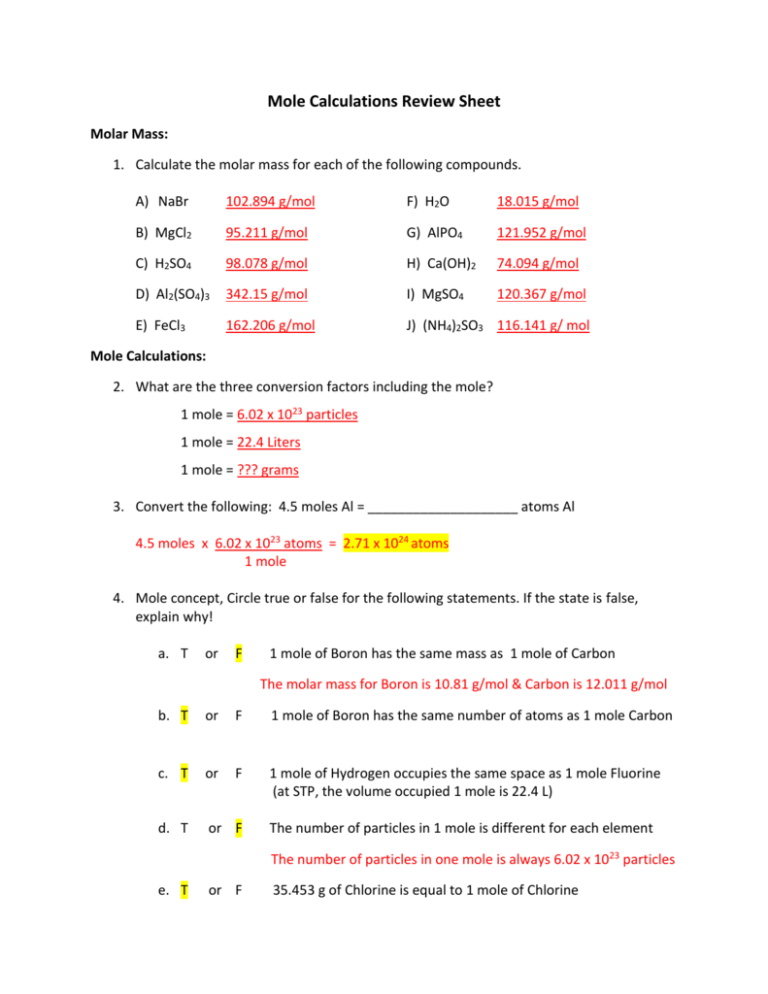
Mole Calculations Review Sheet Molar Mass: 1. Calculate the molar mass for each of the following compounds. A) NaBr 102.894 g/mol F) H2O 18.015 g/mol B) MgCl2 95.211 g/mol G) AlPO4 121.952 g/mol C) H2SO4 98.078 g/mol H) Ca(OH)2 74.094 g/mol D) Al2(SO4)3 342.15 g/mol I) MgSO4 120.367 g/mol E) FeCl3 162.206 g/mol J) (NH4)2SO3 116.141 g/ mol Mole Calculations: 2. What are the three conversion factors including the mole? 1 mole = 6.02 x 1023 particles 1 mole = 22.4 Liters 1 mole = ??? grams 3. Convert the following: 4.5 moles Al = ____________________ atoms Al 4.5 moles x 6.02 x 1023 atoms = 2.71 x 1024 atoms 1 mole 4. Mole concept, Circle true or false for the following statements. If the state is false, explain why! a. T or F 1 mole of Boron has the same mass as 1 mole of Carbon The molar mass for Boron is 10.81 g/mol & Carbon is 12.011 g/mol b. T or F 1 mole of Boron has the same number of atoms as 1 mole Carbon c. T or F 1 mole of Hydrogen occupies the same space as 1 mole Fluorine (at STP, the volume occupied 1 mole is 22.4 L) d. T or F The number of particles in 1 mole is different for each element The number of particles in one mole is always 6.02 x 1023 particles e. T or F 35.453 g of Chlorine is equal to 1 mole of Chlorine 5. How many moles of CO2 are in 6.32 Liters of CO2? 6.32 Liters x 1 mole = 0.282 moles 22.4 Liters 6. How many Liters are in 5.38 x 1024 molecules of O2? 5.38 x 1024 molecules x 1 mole x 6.02 x 1023 molecules 22.4 L = 200. Liters 1 mole 7. Convert the following: 3.2g Na = __________________ atoms Na 3.2 grams x 1 mole x 6.02 x 1023 atoms = 8.38 x 1022 atoms 22.990 g 1 mole 8. What is the mass of 6.43 x 1025 molecules of CCl4? 6.43 x 1025 molecules x 1 mole x 153.823 g = 16,400 g 6.02 x 1023 molecules 1 mole 9. How many grams are in 3.14 x 1023 molecules of Cl2? 3.14 x 1023 molecules x 1 mole x 6.02 x 1023 molecules 70.906 g = 37.0 g 1 mole 10. Convert the following: 62.8g Mg(NO3)2 = ________________ moles Mg(NO3)2 62.8 g x 1 mole = 0.423 moles 148.313 g








