DIATOMICS AND DA BOXES 2015
advertisement

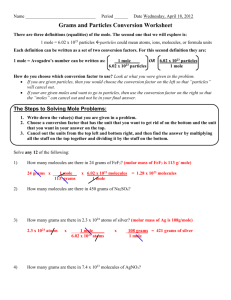
25 point assignment DIATOMICS AND DA BOXES: Due at the Beginning of Class 1. Rewrite BOTH the NAME and the SYMBOL of each Diatomic 2 times (as seen in yellow highlight) Iodine Bromine Chlorine Fluorine Oxygen Nitrogen Hydrogen Iodine Bromine Chlorine Fluorine Oxygen Nitrogen Hydrogen I2 Br2 Cl2 F2 O2 N2 H2 I2 Br2 Cl2 F2 O2 N2 H2 2. Rewrite each of the following DA boxes 5 times (as seen in Green highlight). An example(s) is given next to some of the boxes. Write the example 1 time (as seen in blue highlight) a) Mol/gram box 1 mole molar mass in grams Ex. Nickel (ll) Hydroxide Ni(OH)2 Ni = 1 x 59 O = 2 x 16 1 mole 93 grams 1 mole molar mass in grams 1 mole molar mass in grams 1 mole molar mass in grams 1 mole molar mass in grams H= 2 x 1 Total = 93grams b) Mole/molecule box (no example here) 1 mole 6.02 x 1023 molecules 1 mole 6.02 x 1023 molecules 1 mole 6.02 x 1023 molecules 1 mole 6.02 x 1023 molecules 1 mole 6.02 x 1023 molecules C) Atom /molecule box count subscripts for atoms Ne 1A 1 molecule count subscripts for atoms 1 molecule count subscripts for atoms 1mc N2 2A 1mc H2O 1 molecule count subscripts for atoms 1 molecule count subscripts for atoms 1 molecule 3A 1mc CH4 5A 1mc Fe2(SO4)3 17A 1 “mc”