Semester 2 Review

12-13
IPS SEMESTER 2 REVIEW NAME
Part 1: Define the following terms: (be sure they are in your glossary)
Solute -
Solvent-
Solubility-
Saturated Solution-
Mass-
Matter-
Density-
Volume-
Chemical Change-
Physical Change-
Precipitate-
Filtrate-
Soluble-
Pure Substance-
Mixture-
Part 2: Answer the following questions.
1. What is a characteristic property? Why are they useful?
2. List 6 characteristic properties.
3. How do you calculate the concentration of a solution? What are the units?
4. How does the solubility of a solution differ from the concentration? What are the units?
1
12-13
5. List 3 ways to increase the rate of dissolving for solids.
6. How does increasing the temperature of a solution affect the solubility of solids? Of gases?
7. List 6 tests that can be done to help identify an unknown substance.
8. How can you separate a mixture of liquids? What property must the liquids differ in for this procedure to work?
9. How can you separate a mixture of a soluble and an insoluble solid?
10. How can you separate a mixture of two insoluble solids? What property must they differ in?
11. How do you know when you have reached the boiling point or melting point of a substance?
12. What procedure can you use to change a gas back into a liquid? What part of your distillation setup did this?
2
12-13
13. What is the procedure for finding the density of
A rock?
A cube?
A cork stopper?
A liquid?
14. Is the density of a substance dependent only on the mass of the sample? Only on the volume of a sample?
Explain.
15. What observation should you record when testing flammability of a substance?
16. How can you determine of you have a pure substance or a mixture of substances?
3
12-13
Part 3: Use the data table to complete the following solubility calculations. Show your calculations.
17. Evaporating dish and watch glass
Evaporating dish, watch glass and solution
Evaporating dish, watch glass and solid
= 26.43 g
= 29.21 g
= 28.62 g
Solubility =
2
1
Mass of
3
Mass of
Solid evaporating evaporation
(Column 2 – Column 1) dish and watch dish, watch glass and
Mass of Solution (g) glass (g) solution (g)
17.
Solid
4
Mass of evaporation dish, glass and solid (g)
5
(Column 4 – Column 1)
Mass of Solid (g)
17.
Solid
18.
6
(Col 3 – Col 5)
Mass of
Solvent (g)
7
(Col 7 = Col 6)
Volume of
Solvent/Water
(cm 3 )
8
(Col 5/Col 7)
Concentration
(g/cm 3 )
9
(Col 8 x 100)
Solubility (g/100 cm 3 )
4
12-13
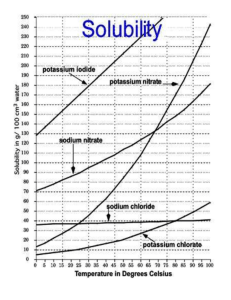
Part 4: Solubility Graph
18. What is the solubility of each of the following solids at 50 °C?
Sodium Nitrate
Potassium Nitrate
Sodium Chloride
Which is most soluble?
Which is least soluble?
19. Are the following solutions saturated or unsaturated?
60 g/100 cm 3 of sodium nitrate at 35 °C?
180g/100cm 3 of potassium nitrate at 80 °C?
75g/100 cm 3 of sodium nitrate at 25 °C?
20. At what temperature will 60g of potassium nitrate dissolve in 100 mL of water?
21. If the solution in question 21 is cooled to 20°C, how much potassium nitrate will stay dissolved?
How much will then precipitate out?
22. If you put 20g of Sodium Nitrate in 20 mL of water and heat it, at what temperature will it all dissolve?
23. At 80 °C, how many grams of potassium nitrate will you need to make a perfectly saturated solution?
24. The best way to describe a solution of 120g/100 cm 3 of sodium nitrate at 40 °C is-
25. At what temperature are the solubilities of potassium nitrate and sodium nitrate the same?
26. At what temperature will equal amounts of sodium chloride and potassium nitrate dissolve?
Unsaturated. or Perfectly saturated. or Saturated with undissolved solid (precipitate)
27. You make a solution which consists of 30g of potassium nitrate in 50 mL of water at 60 °C, how much more solute do you need to add to have a perfectly saturated solution
5
12-13
Part 5: Fractional Distillation
28. Sketch a temp-time curve that might be obtained from distilling a mixture of 3 liquids with the following boiling points: 55°C, 75°C, and 85°C.
Draw vertical lines where you would switch collection tubes so that you can separate the mixture into pure fractions.
Number the fractions.
How many fractions would you have?
Which fractions contain mixtures?
Which fractions contain pure substances?
29. How would the density of the mixtures compare to that of the pure substances in the mixture?
30. If you redistilled one of the test tubes that contains a mixture, how many plateaus would you expect to see?
31. When using fractional distillation to separate a mixture of substances such as petroleum/crude oil, the substances must differ in what characteristic property?
6
12-13
Part 6: Acids and Bases
33. How is an acid different from a base?
34. What is the pH range for acids?
35. What is the pH range for bases?
36. What is the pH of a neutral solution?
37. An acid would turn each of the following indicators what color?
Litmus-
Phenol Red-
Bromthymol Blue-
Phenolphthalein-
7
12-13
38. A base would turn each of the following indicators what color?
Litmus-
Phenol Red-
Bromthymol Blue-
Phenolphthalein-
39. How would a solution with a pH of 3 be different from a solution with a pH of 8? pH 3 solution has _________ x (as many / fewer) H + ions as a solution with a pH of 8. pH 3 solution has _________ x (as many / fewer) OH ions as a solution with a pH of 8.
40. What would you add to each of the following to neutralize it?
A strong base-
A weak base-
A strong acid-
A weak acid-
8